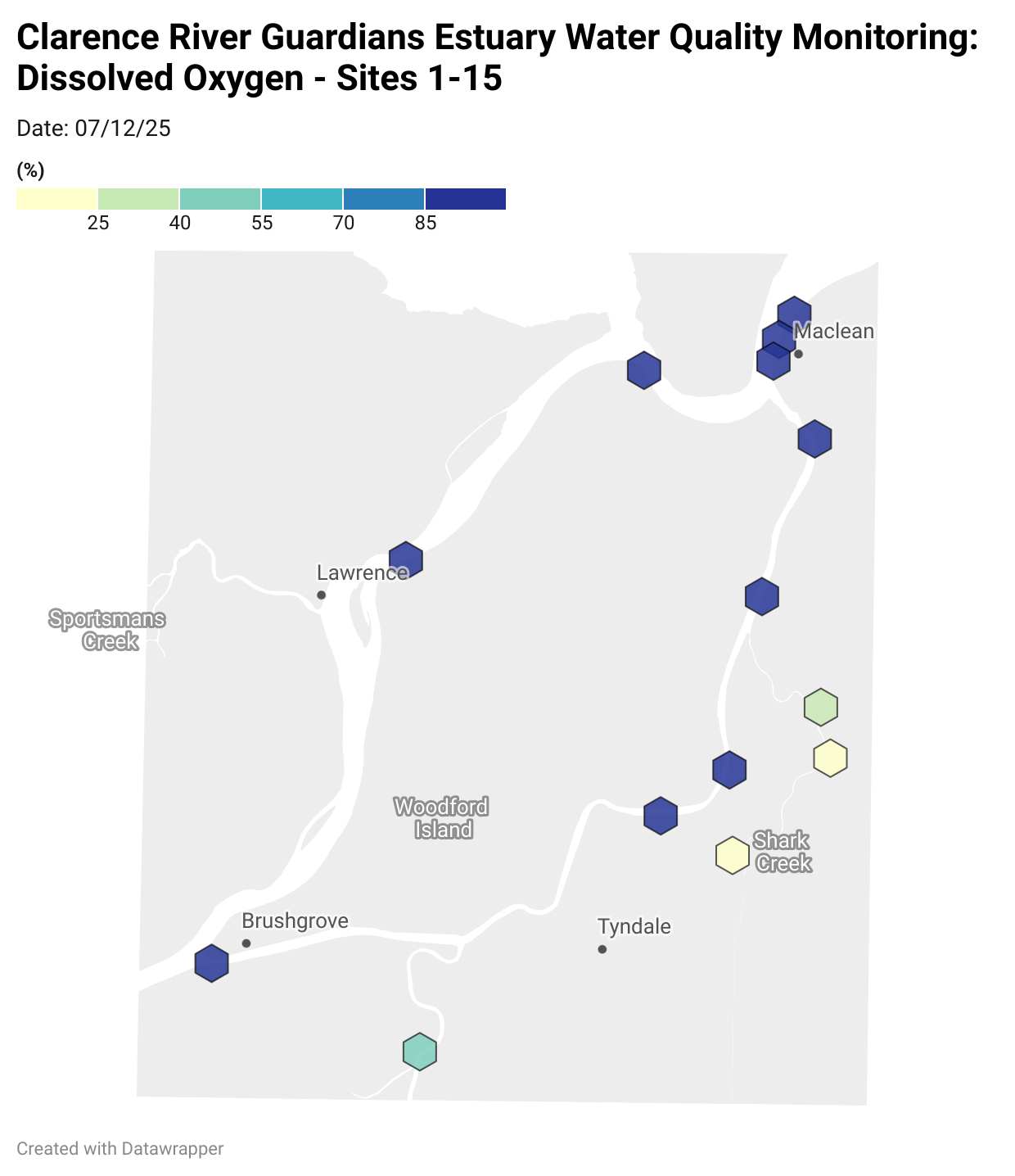
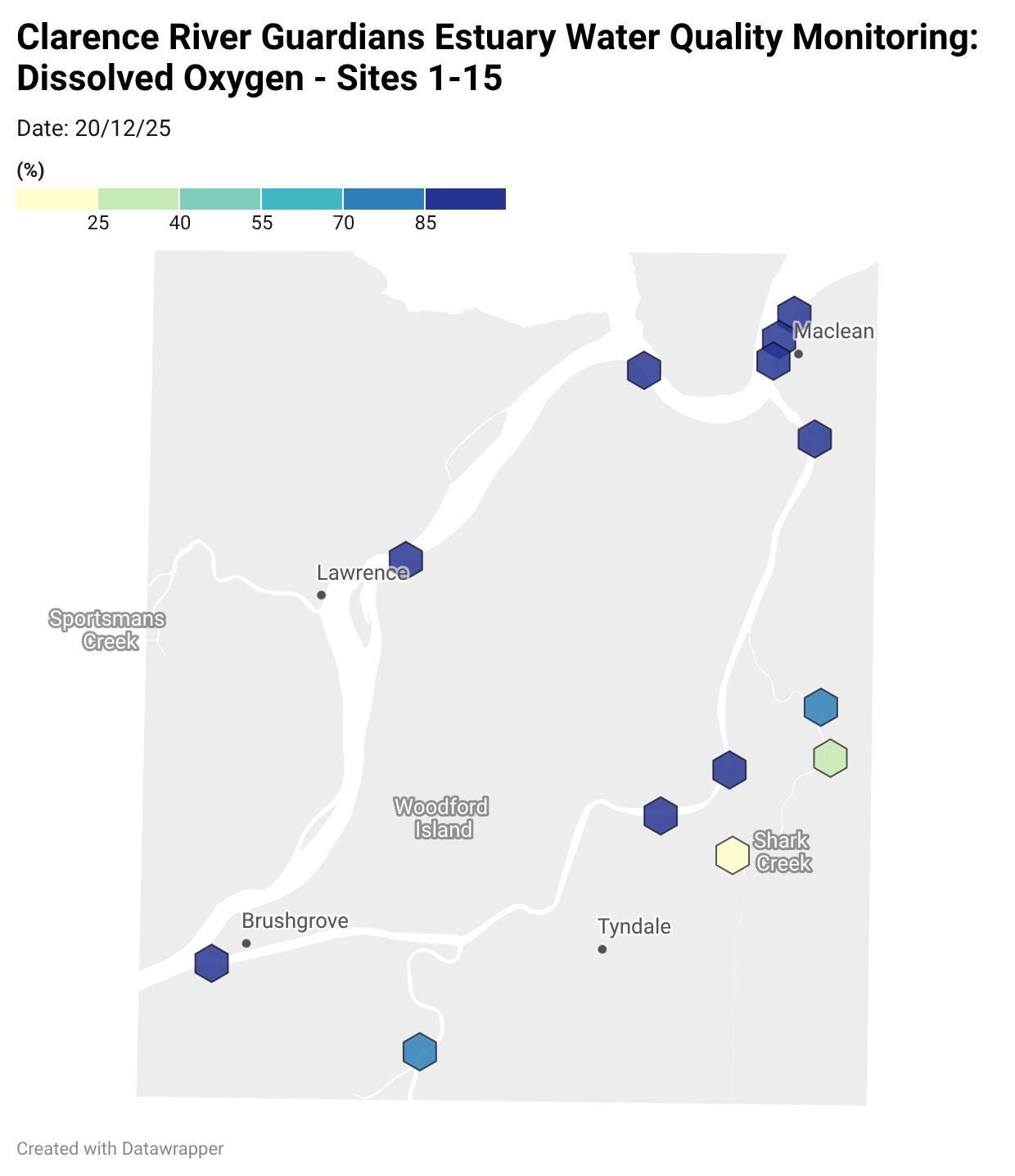
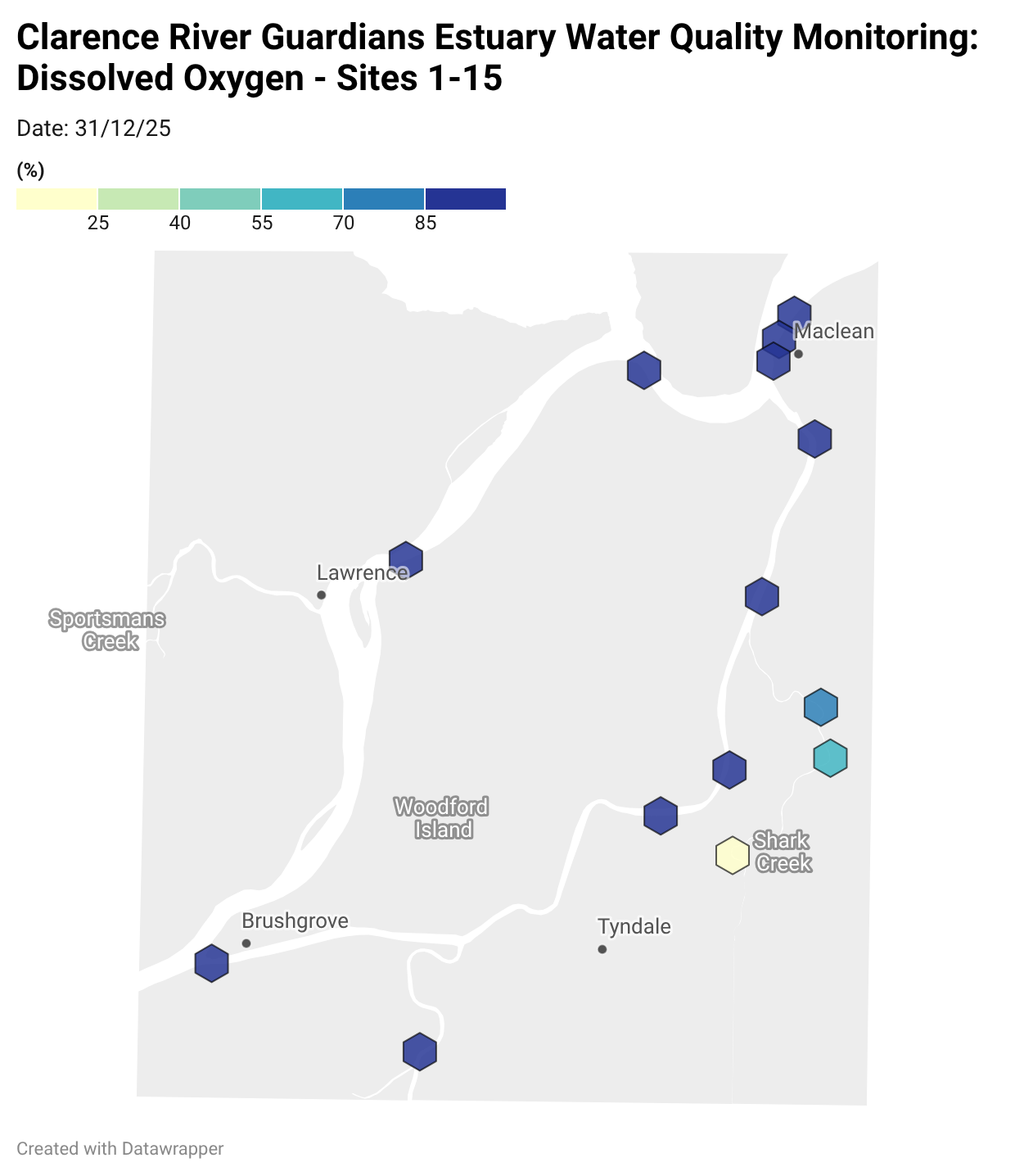
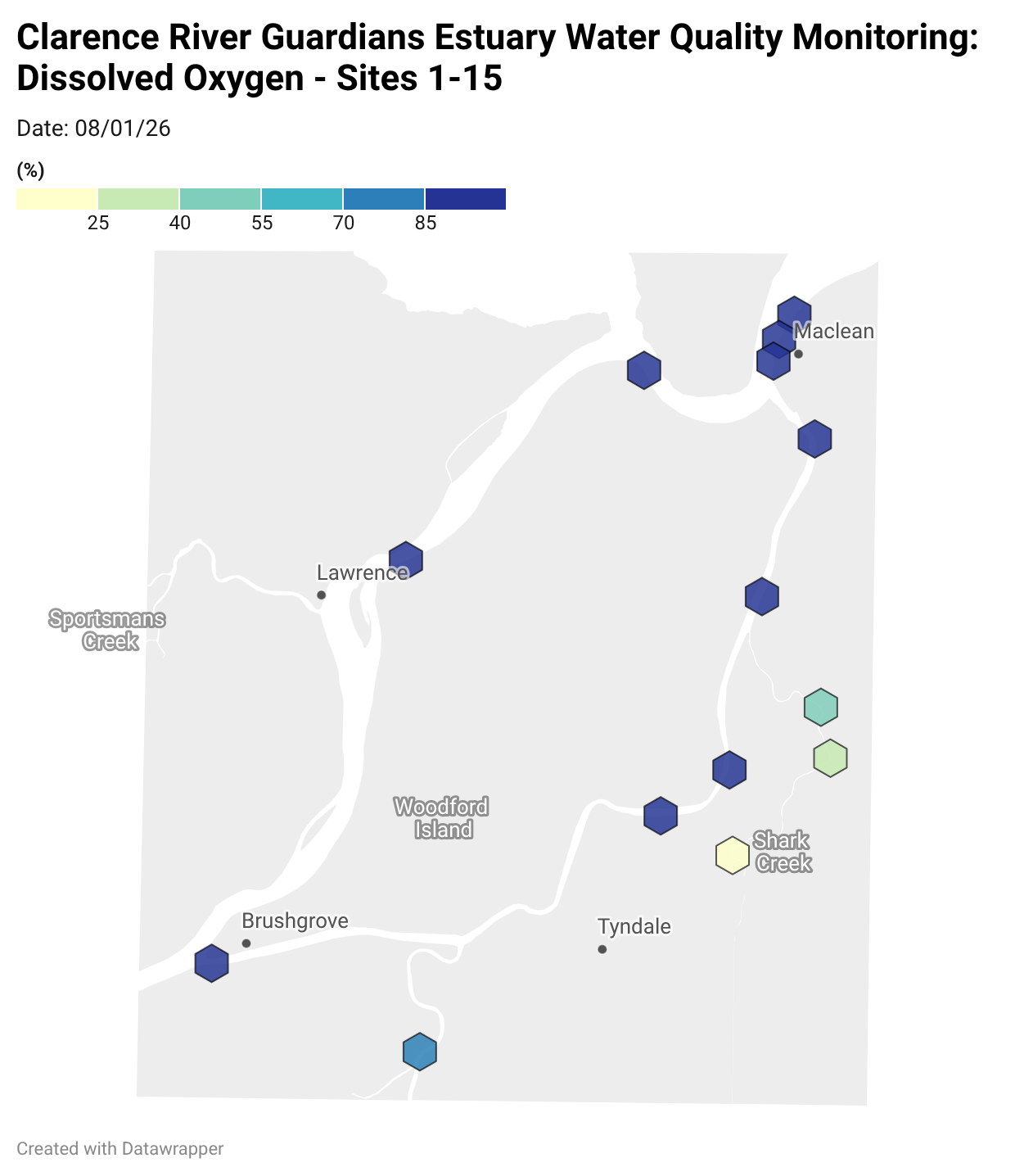
Where are we monitoring?
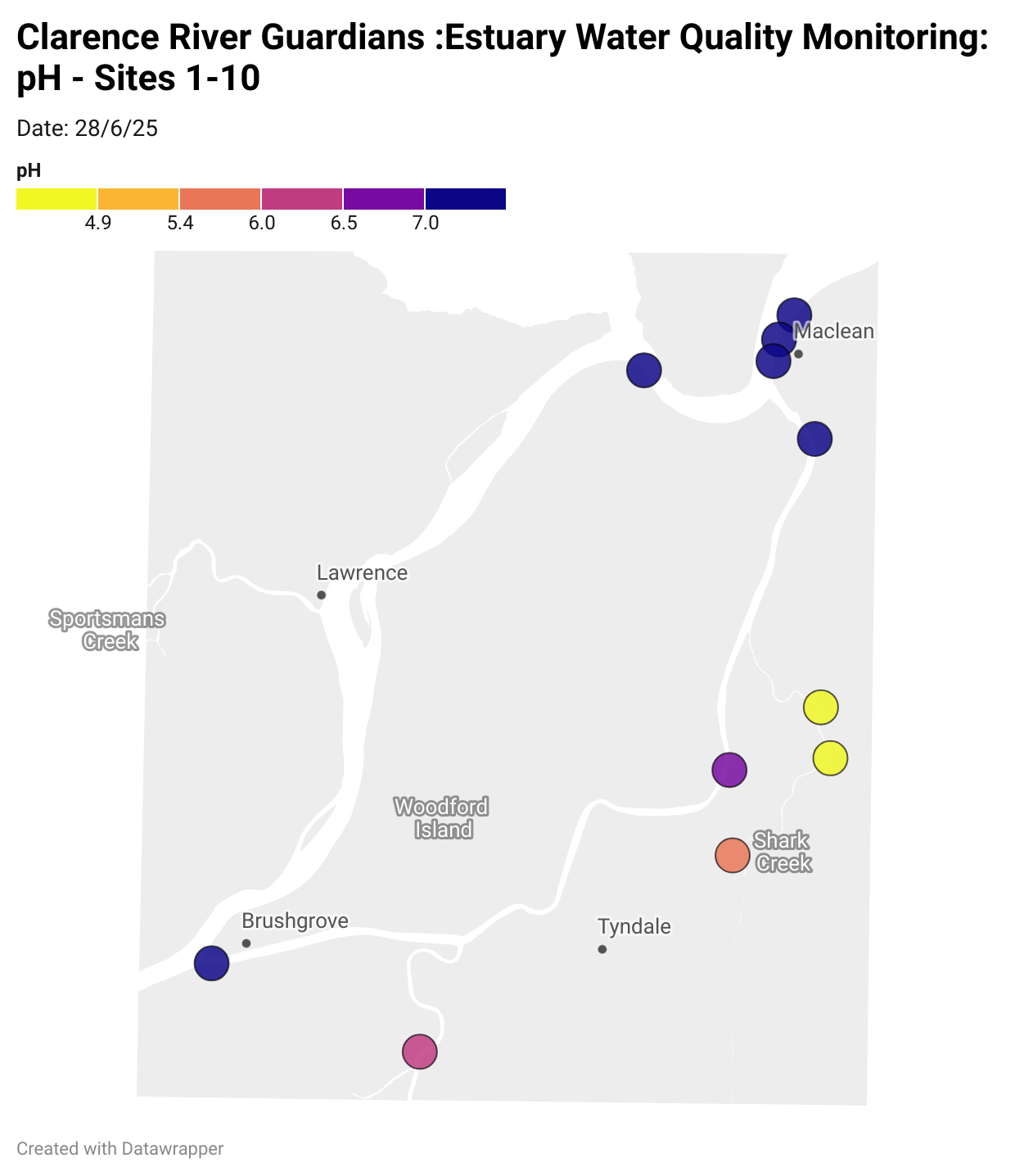
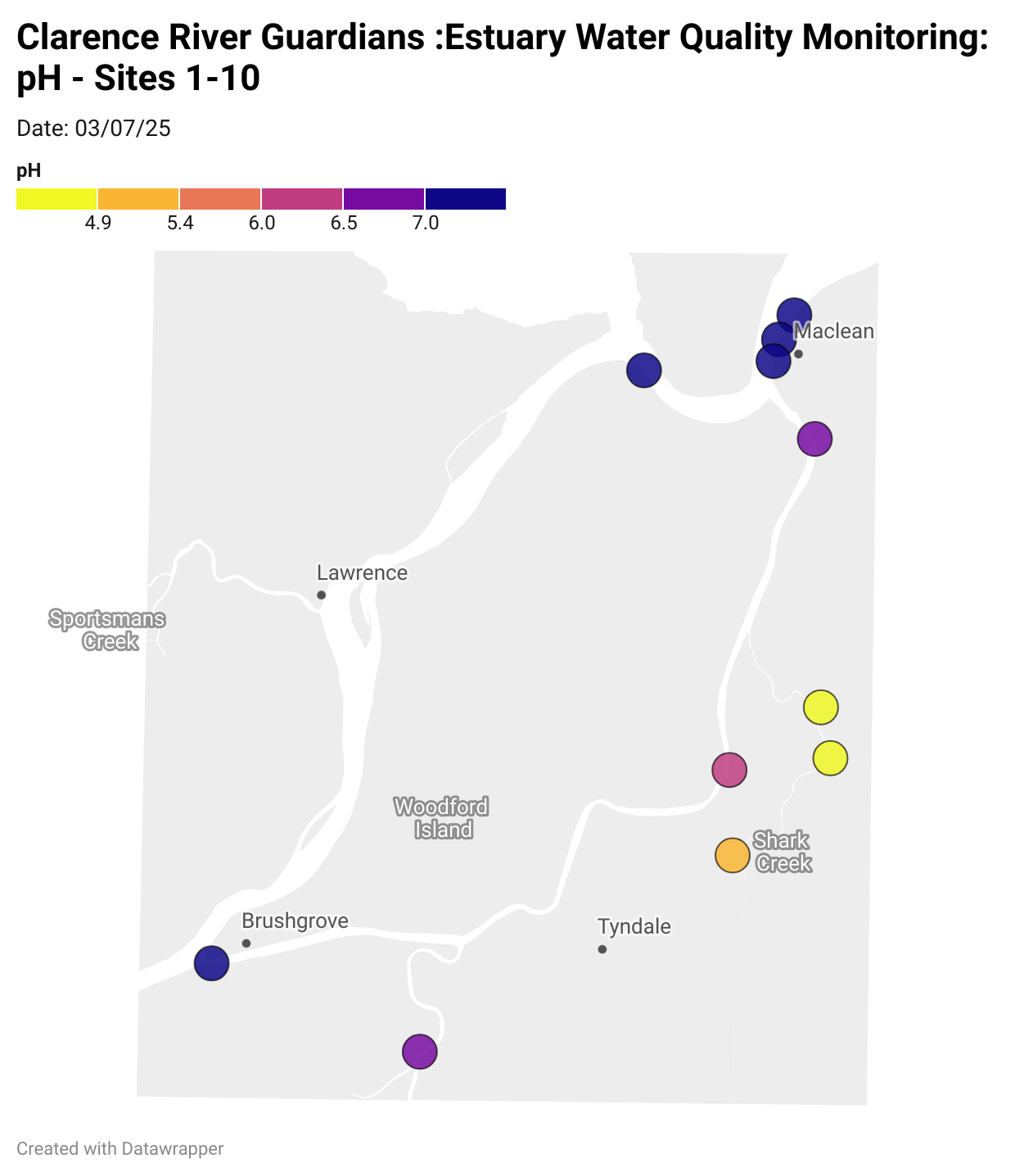
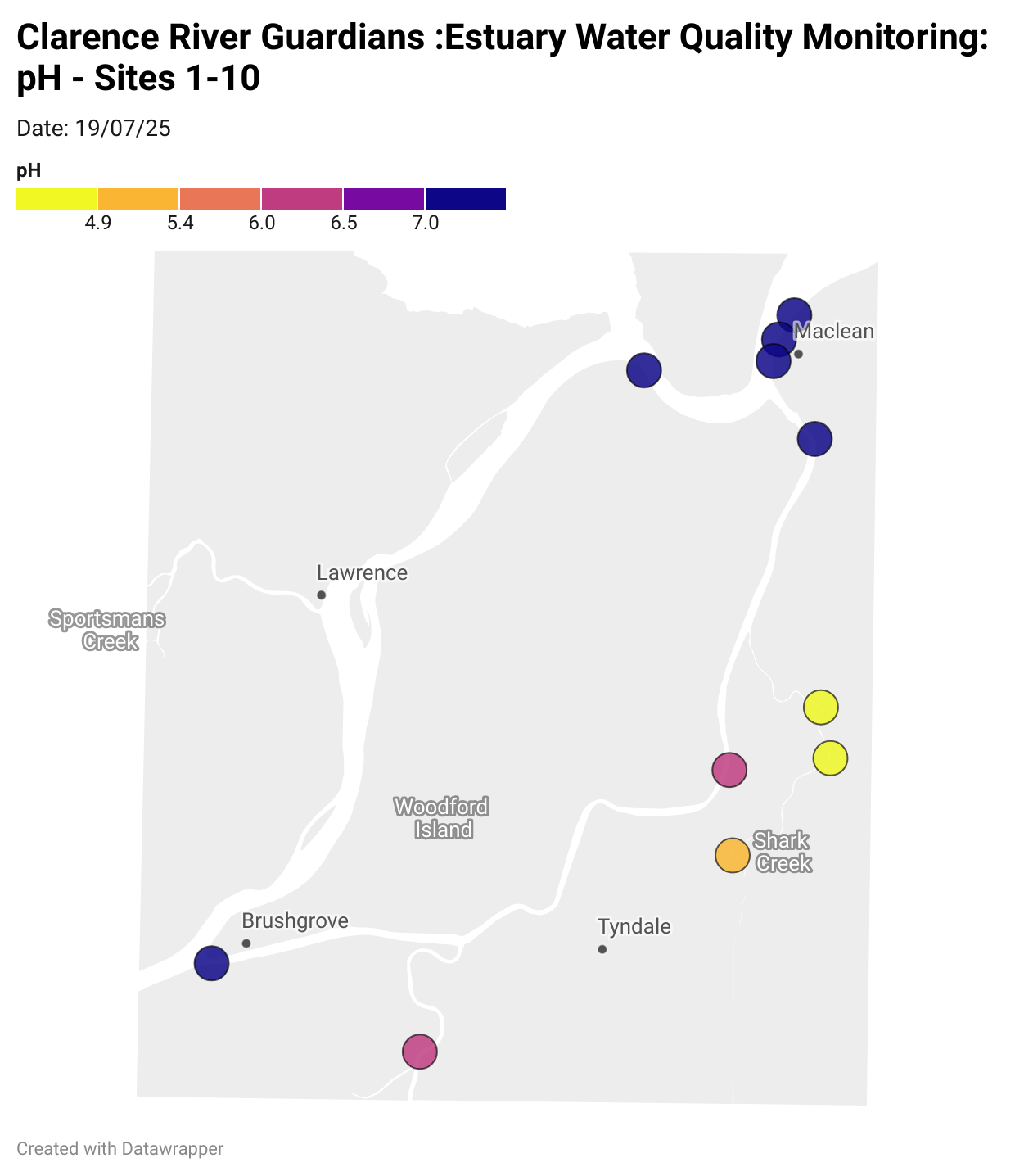
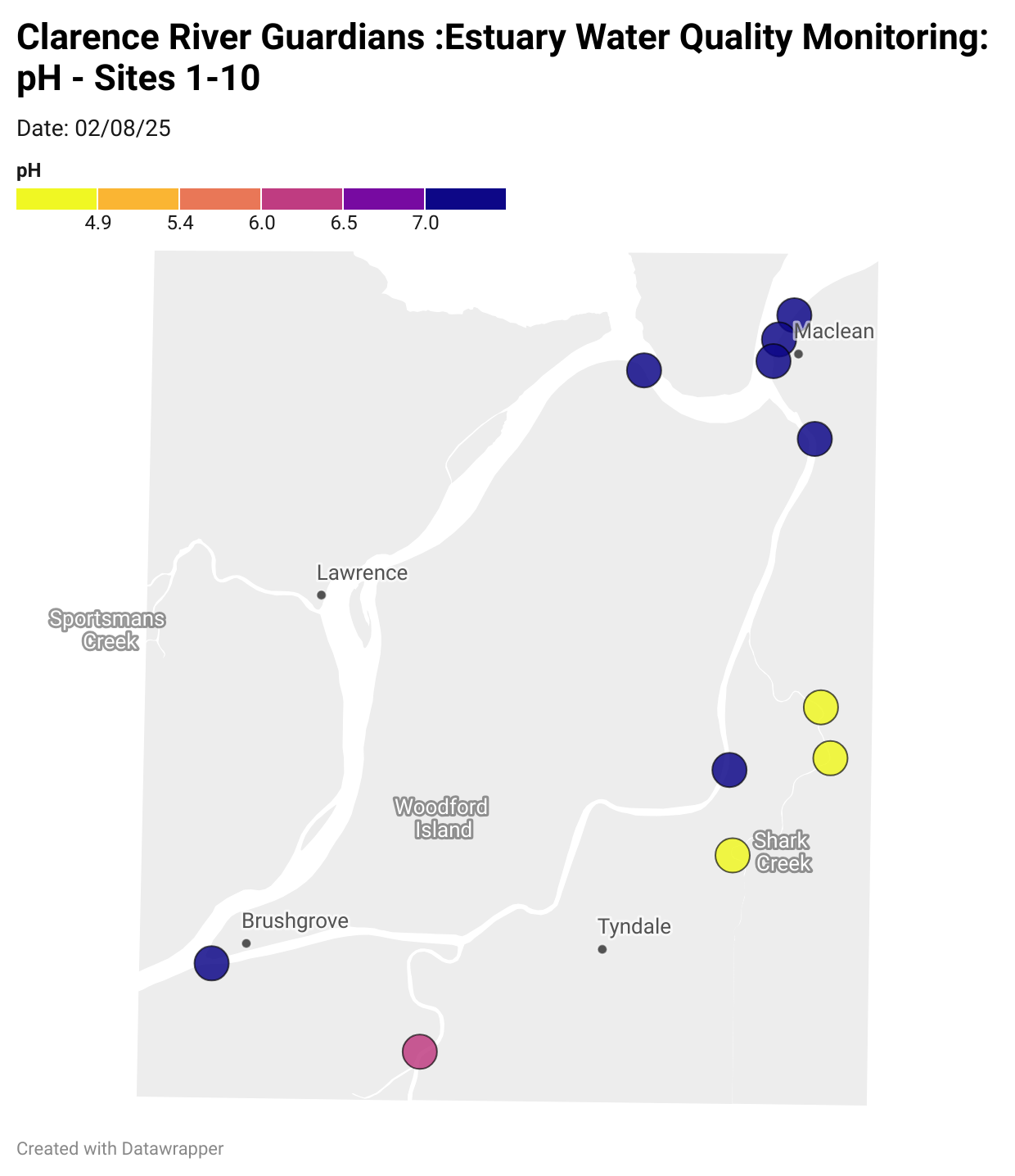
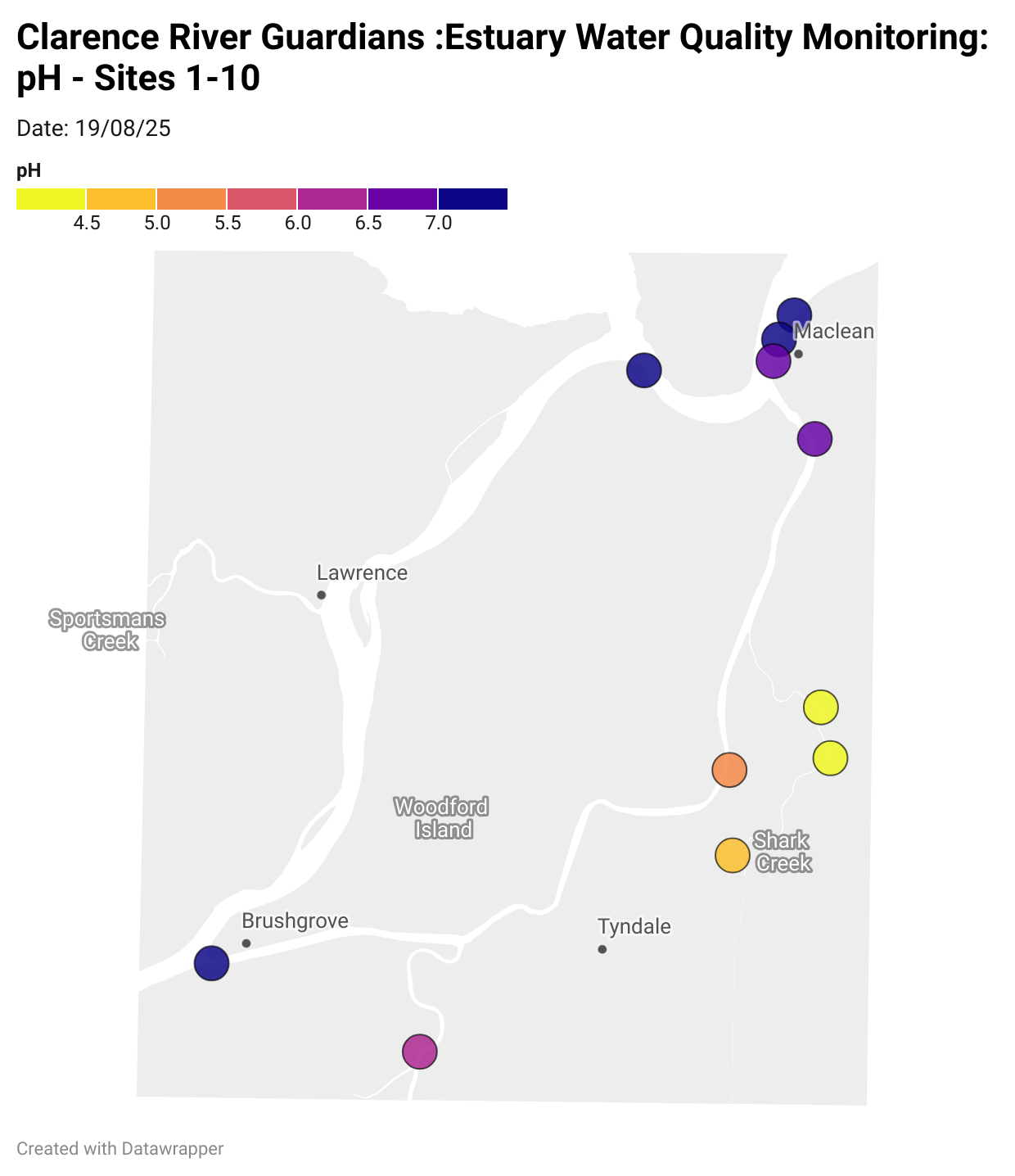
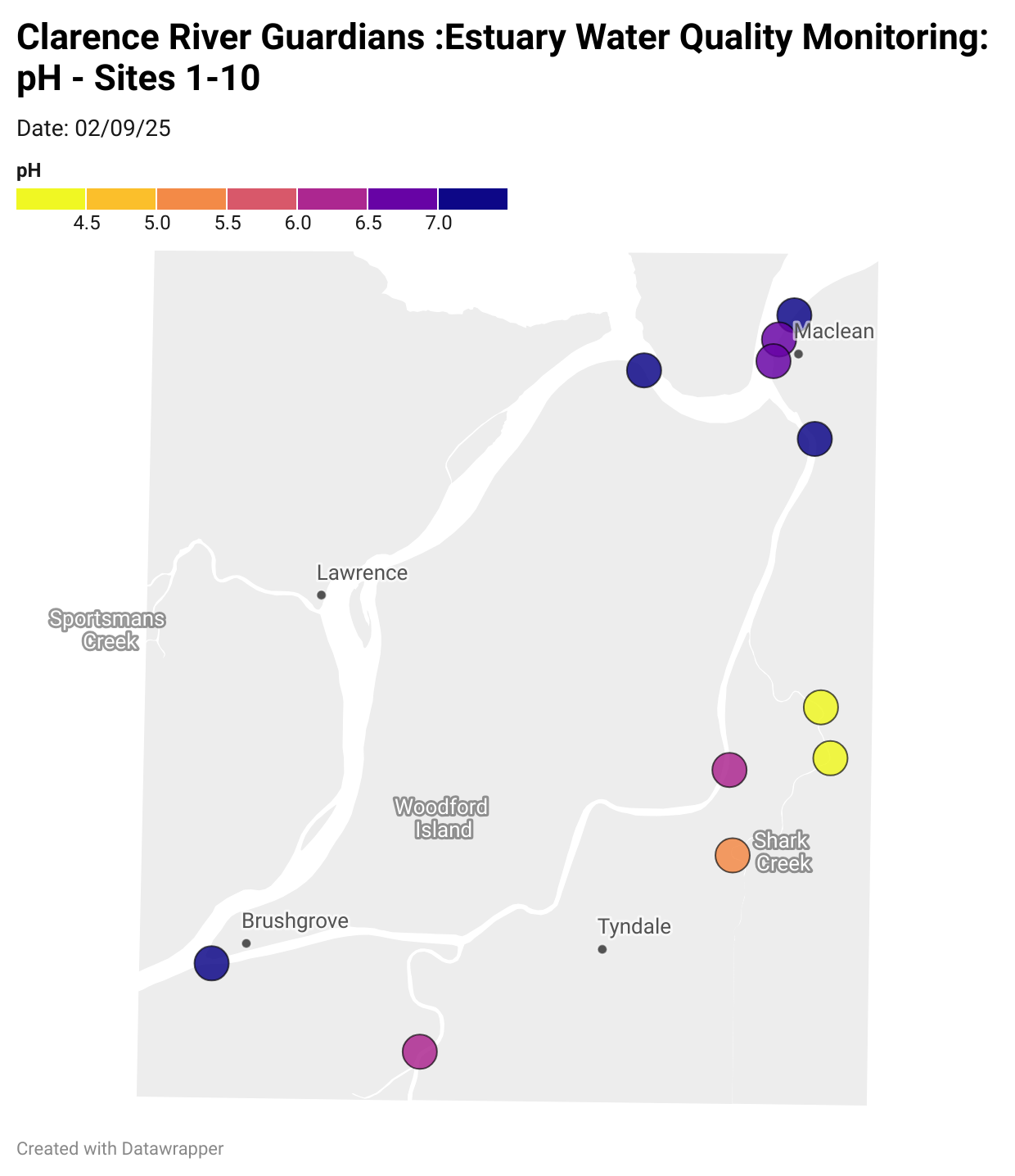
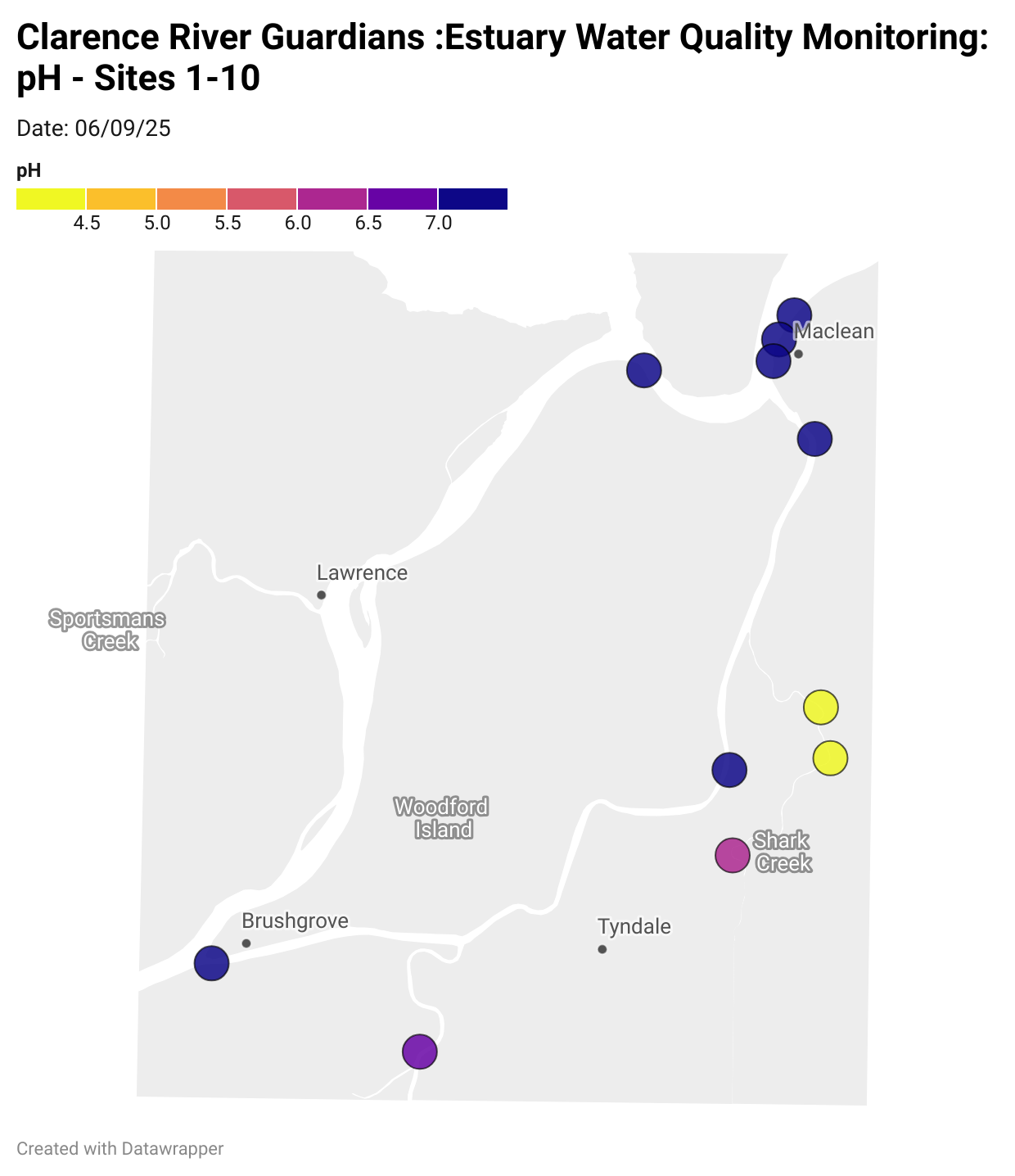
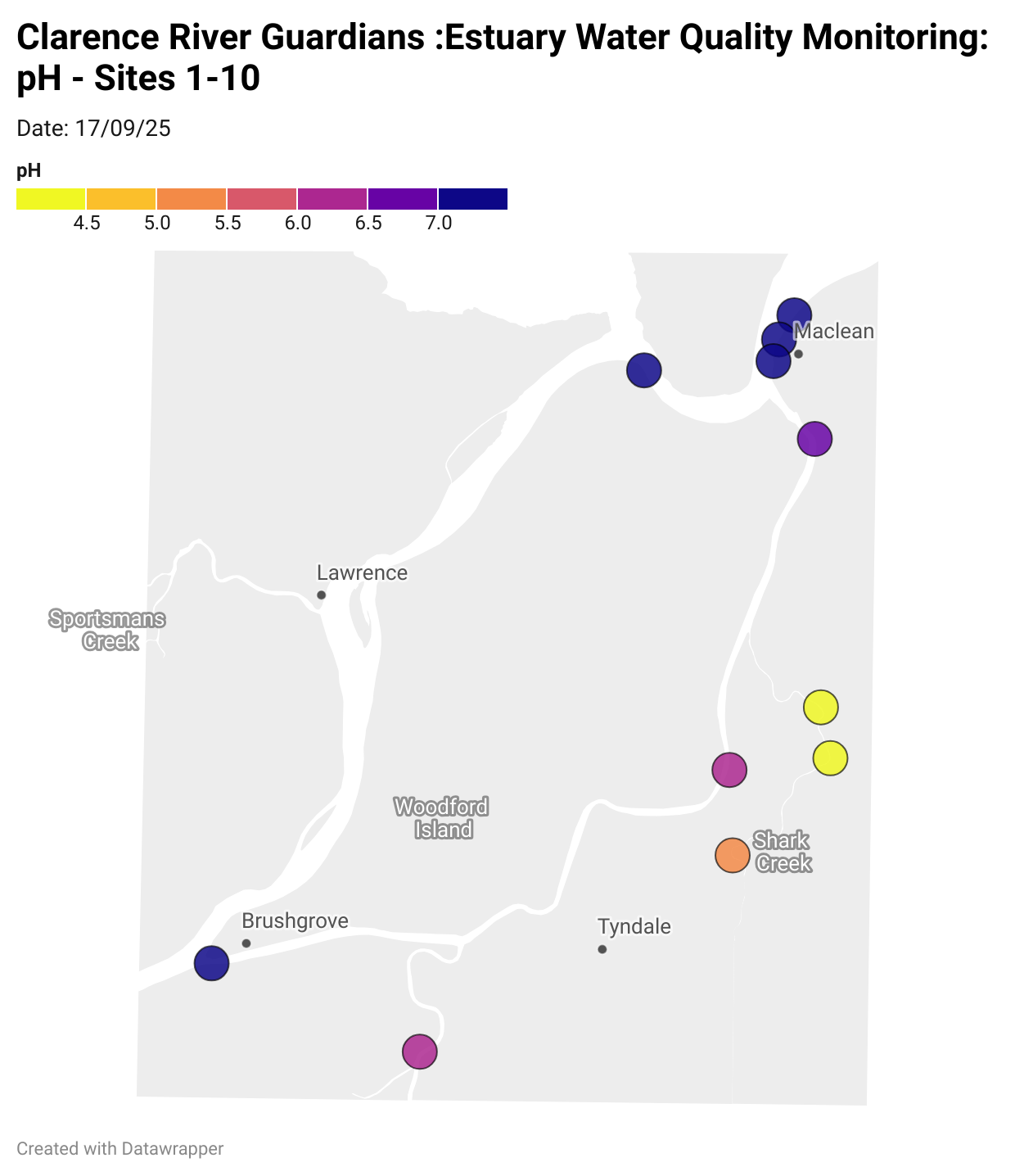
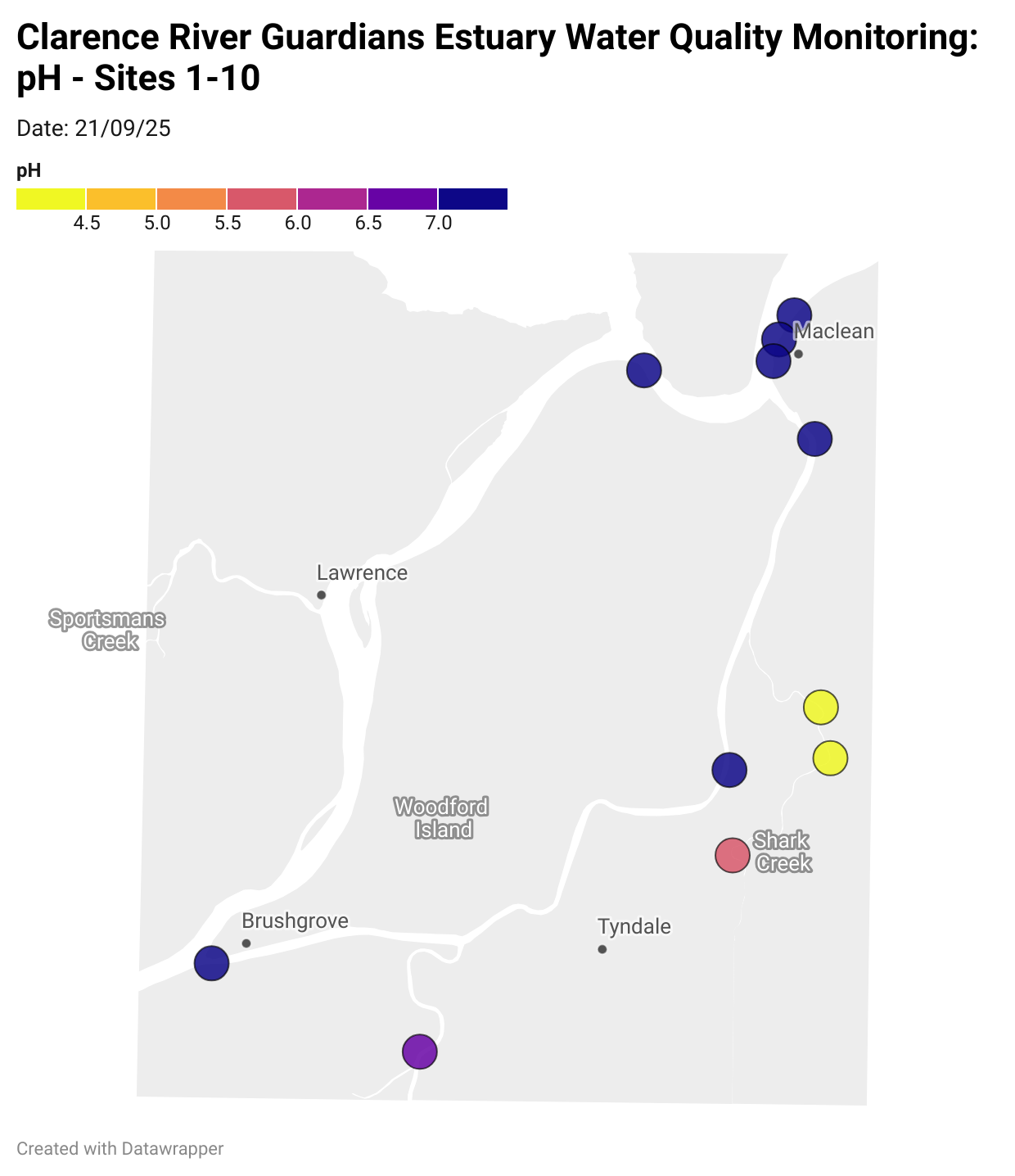
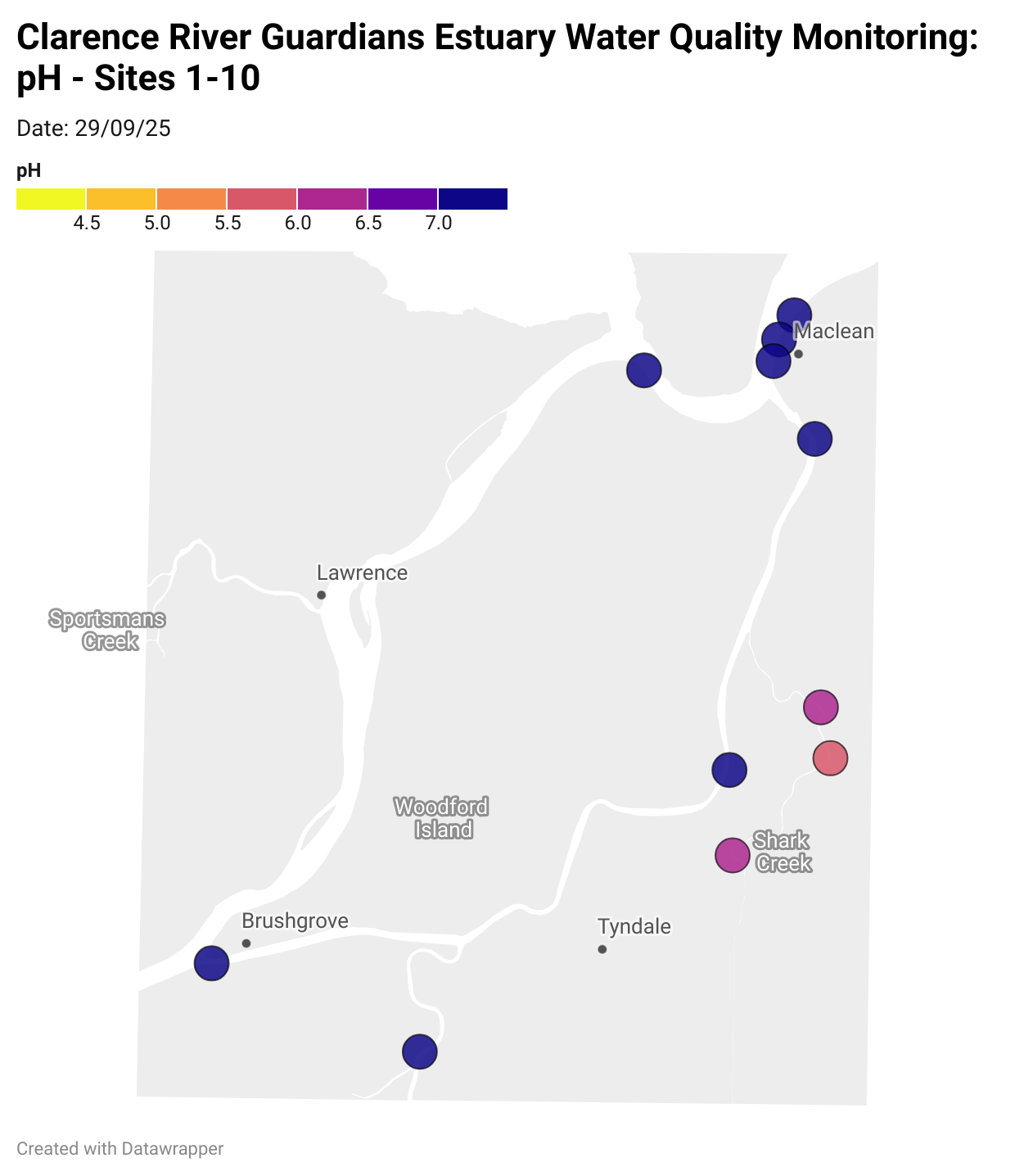
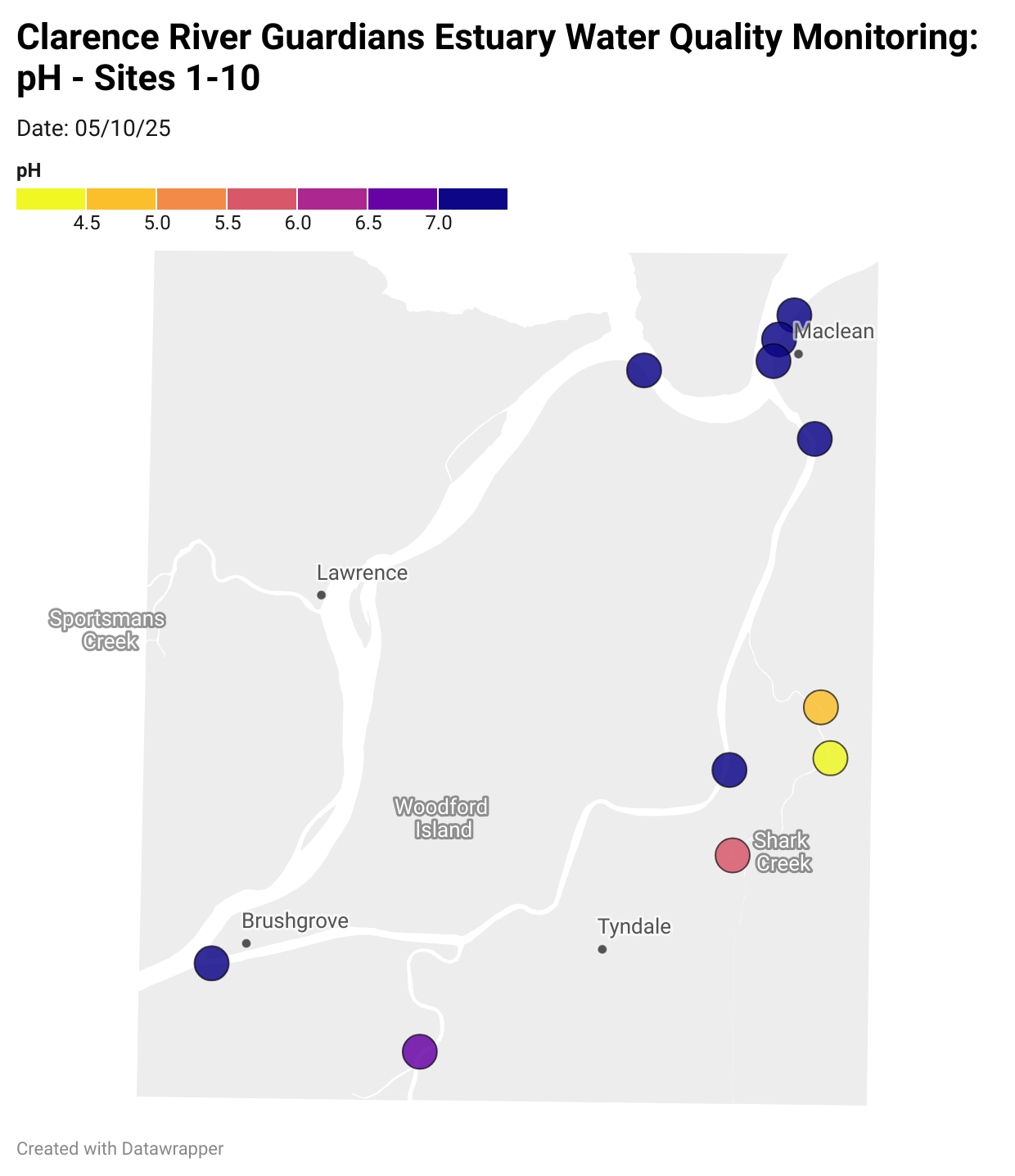
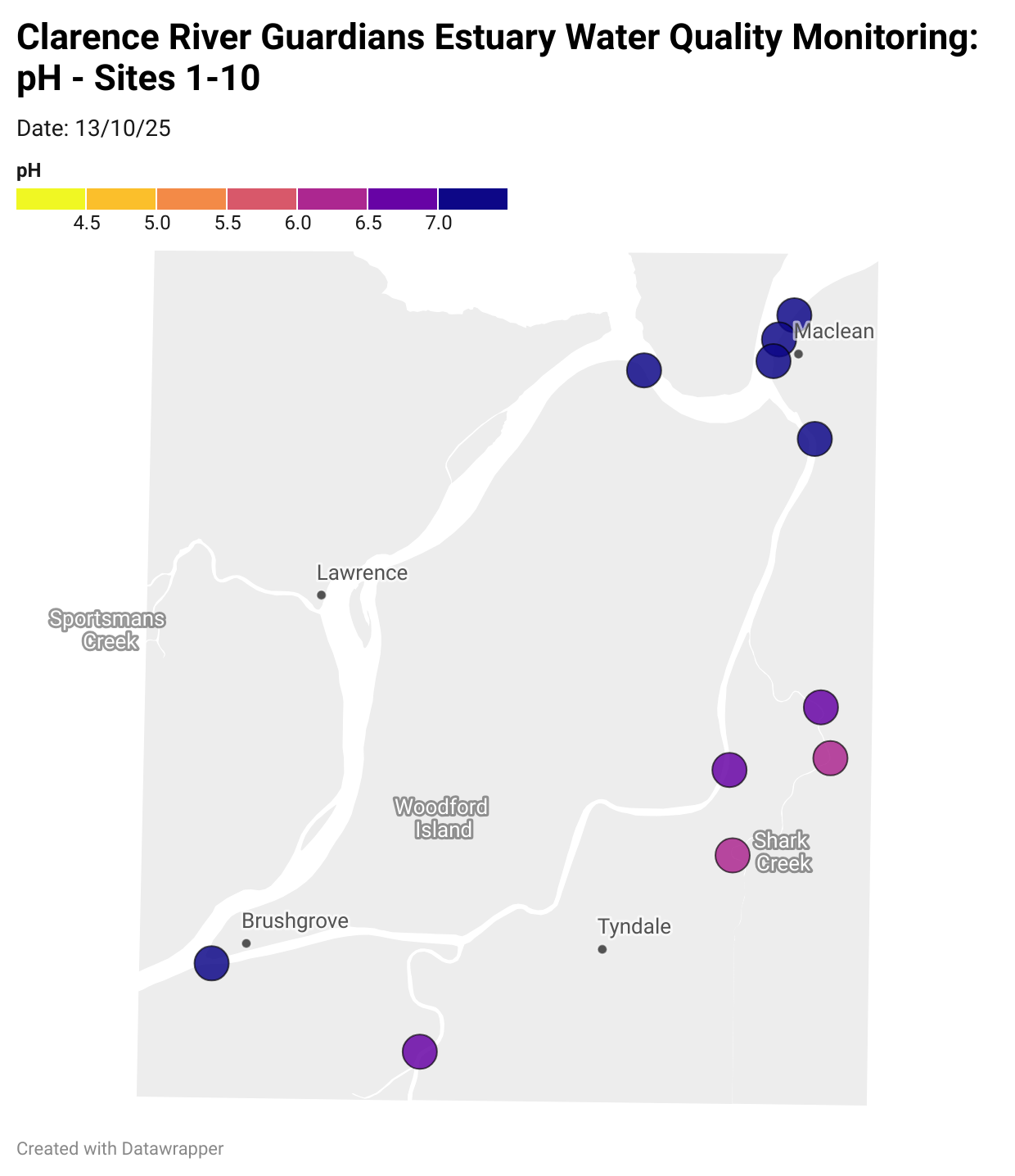
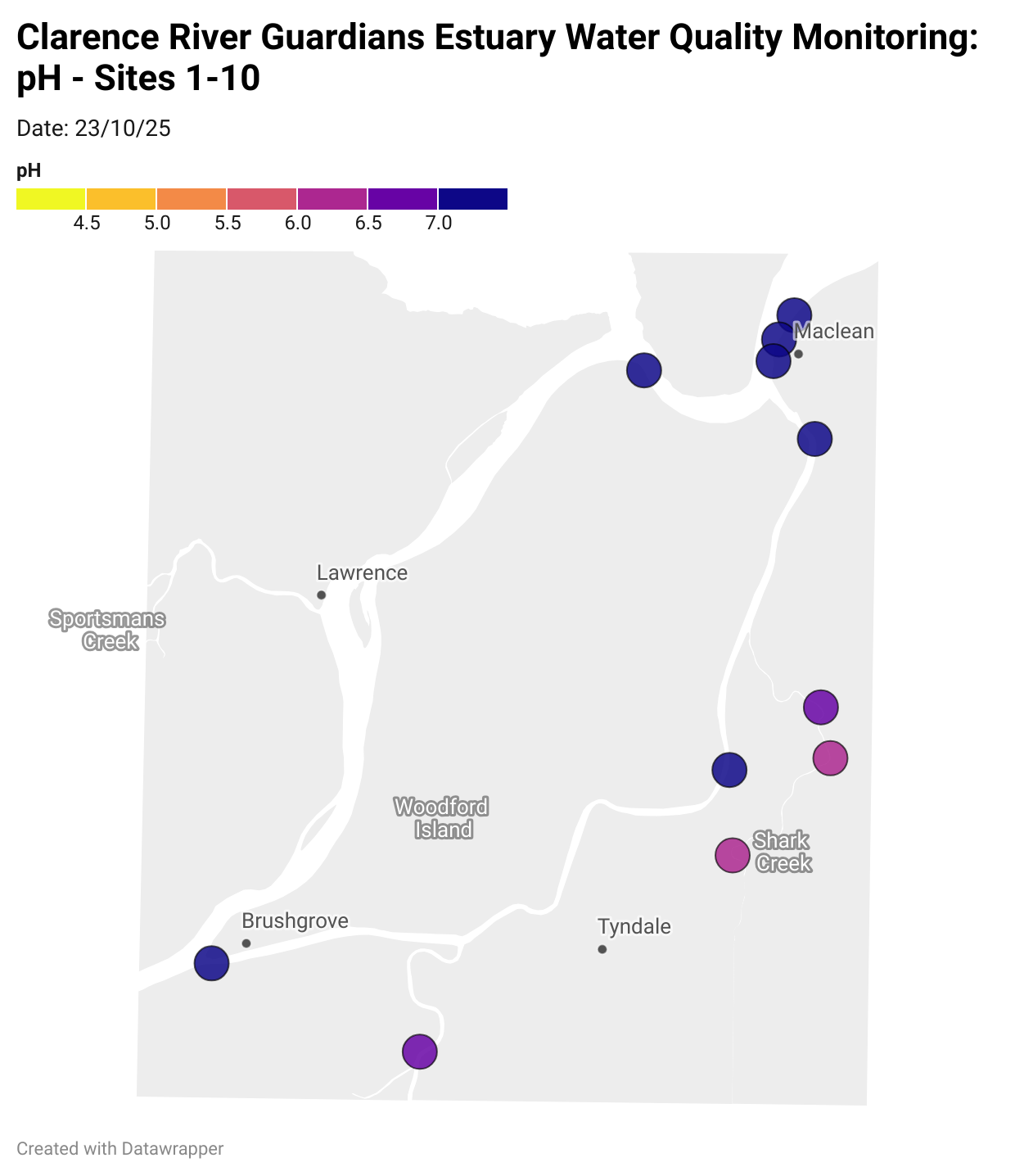
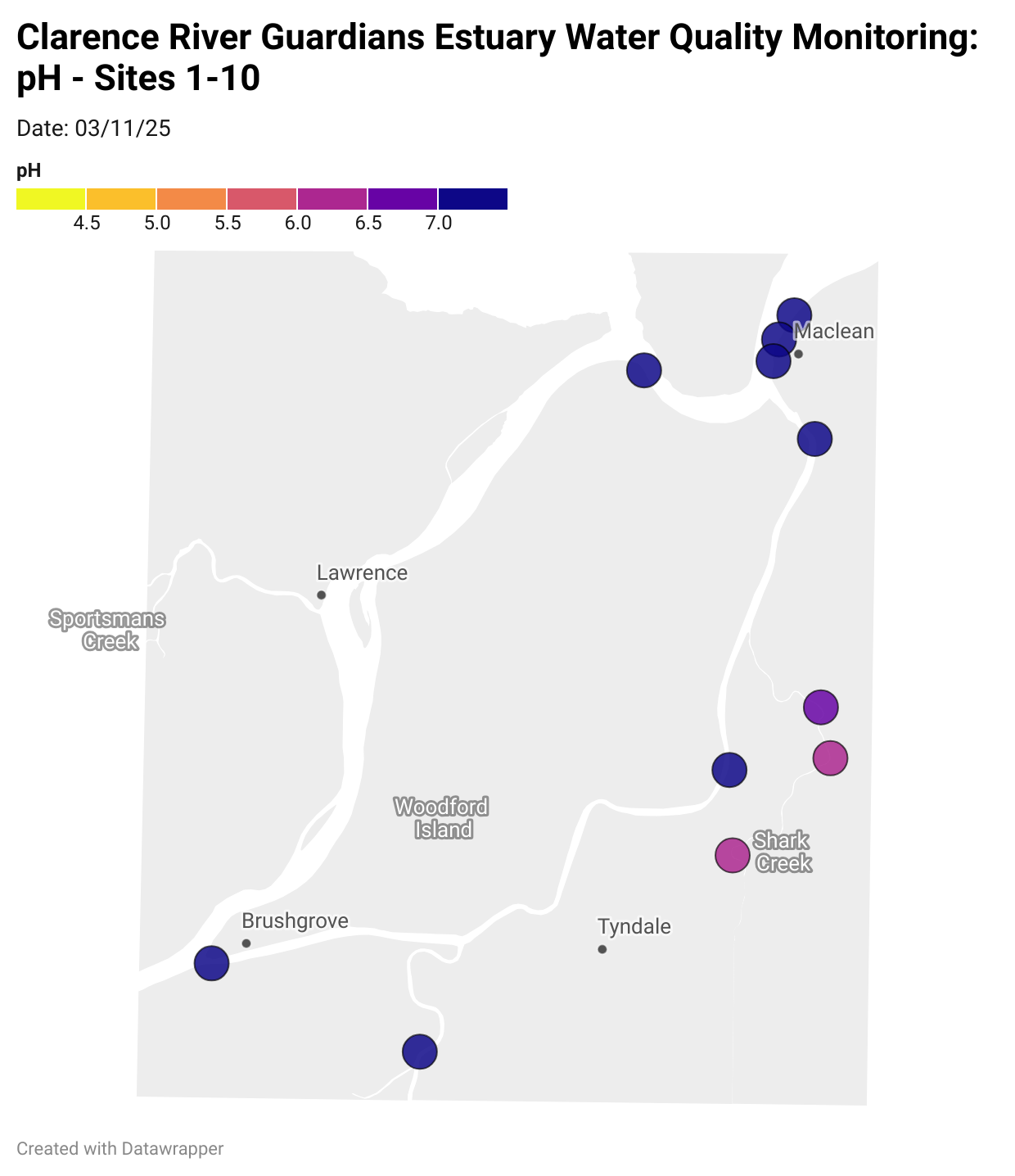
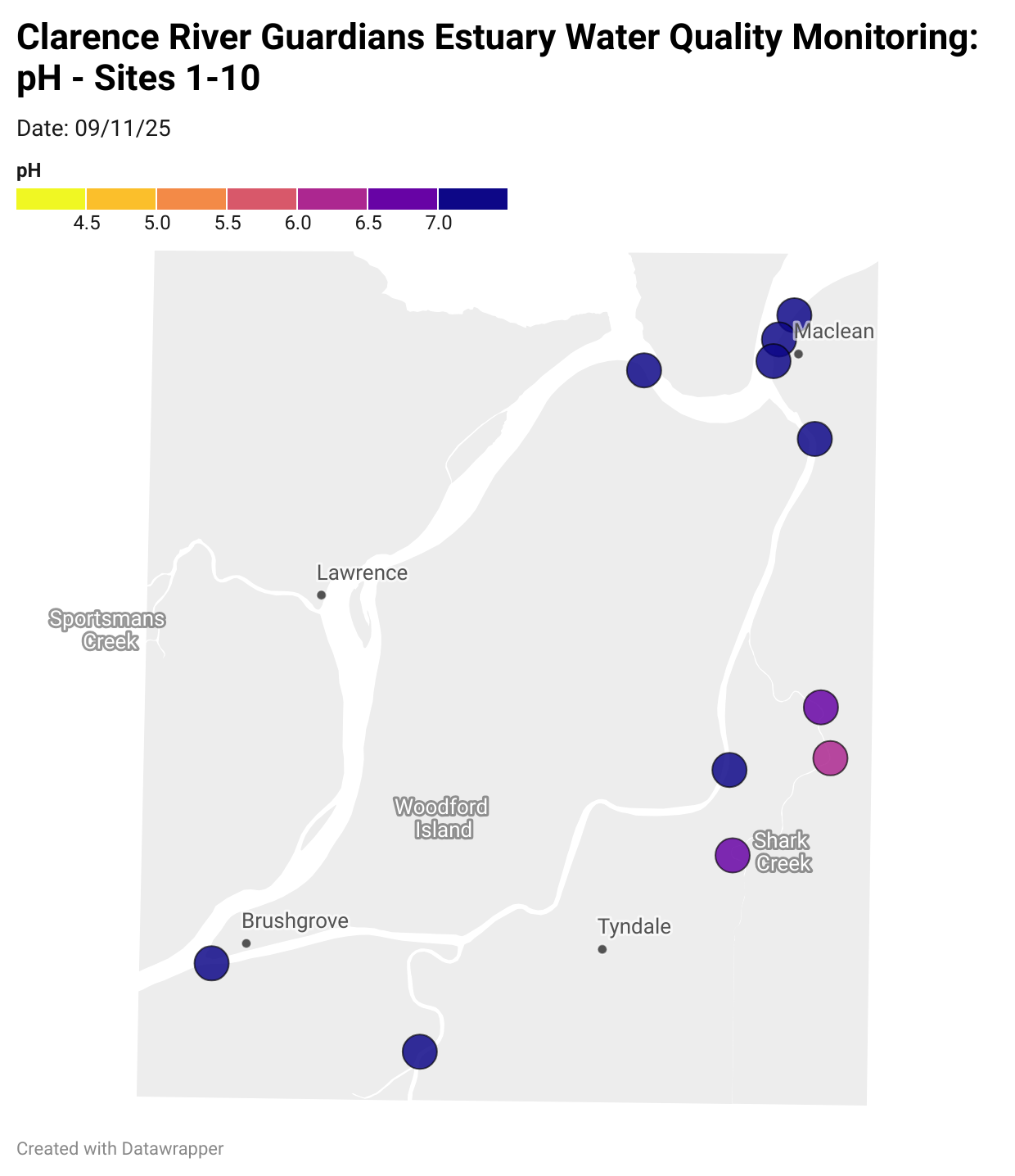
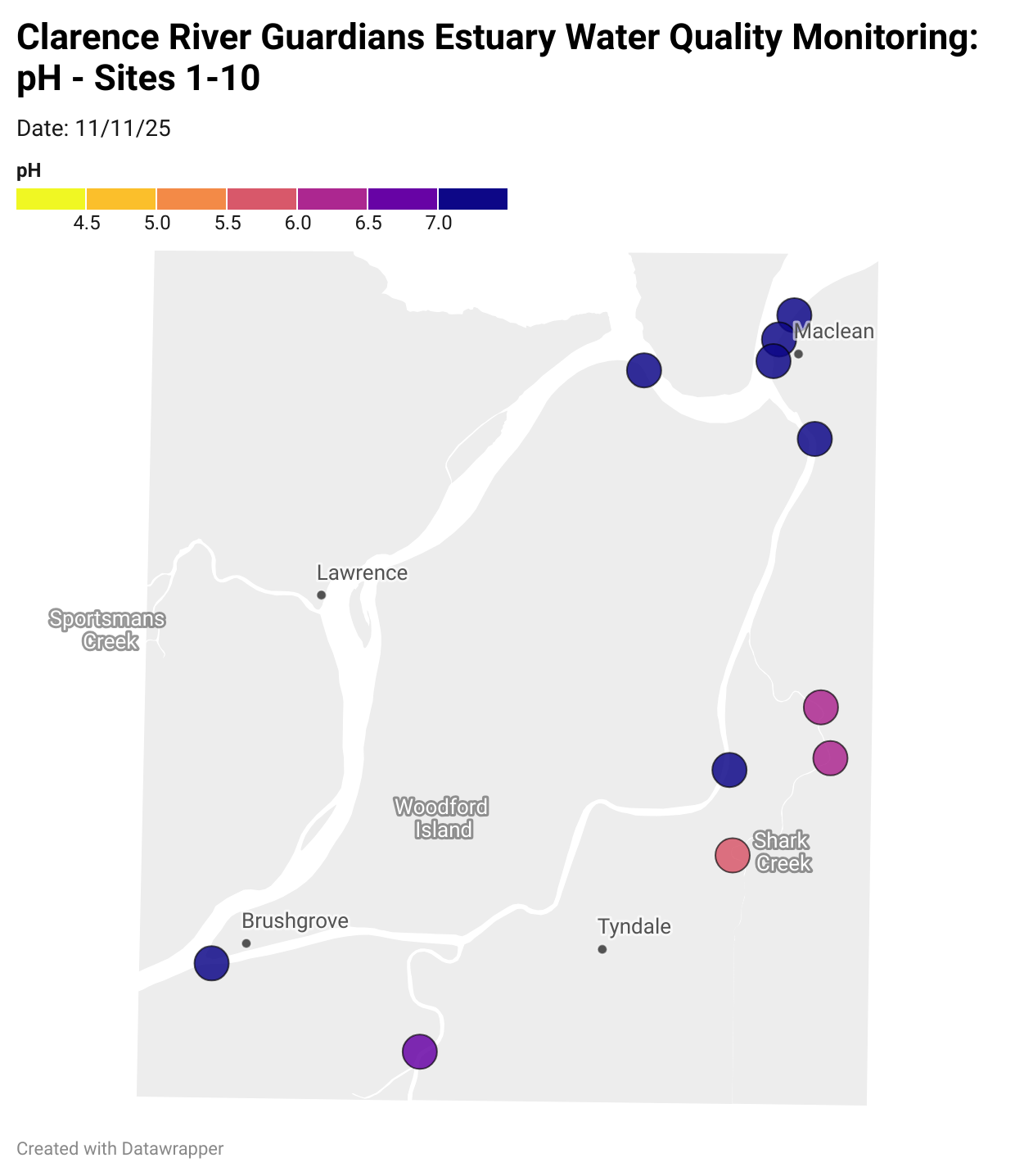
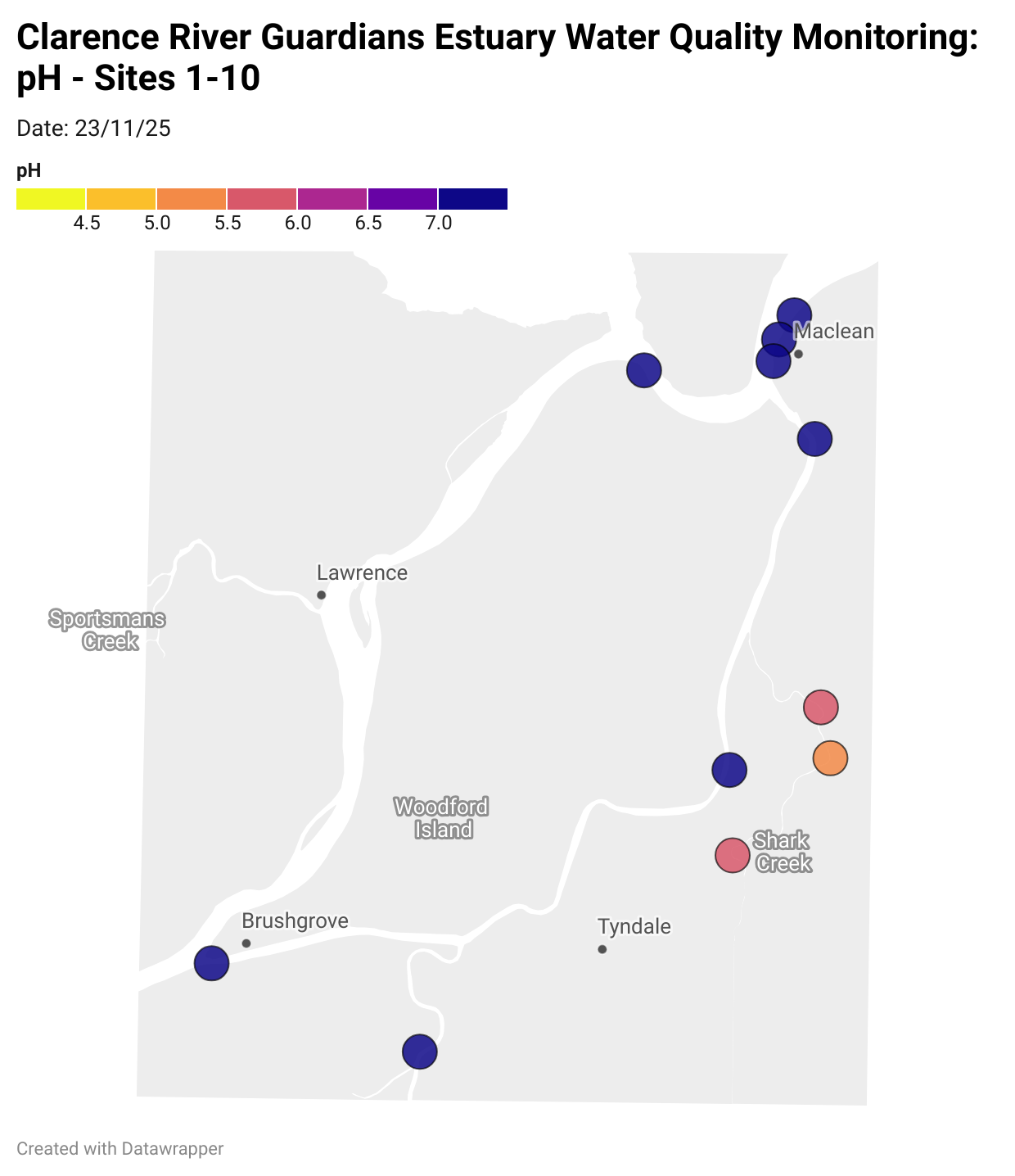
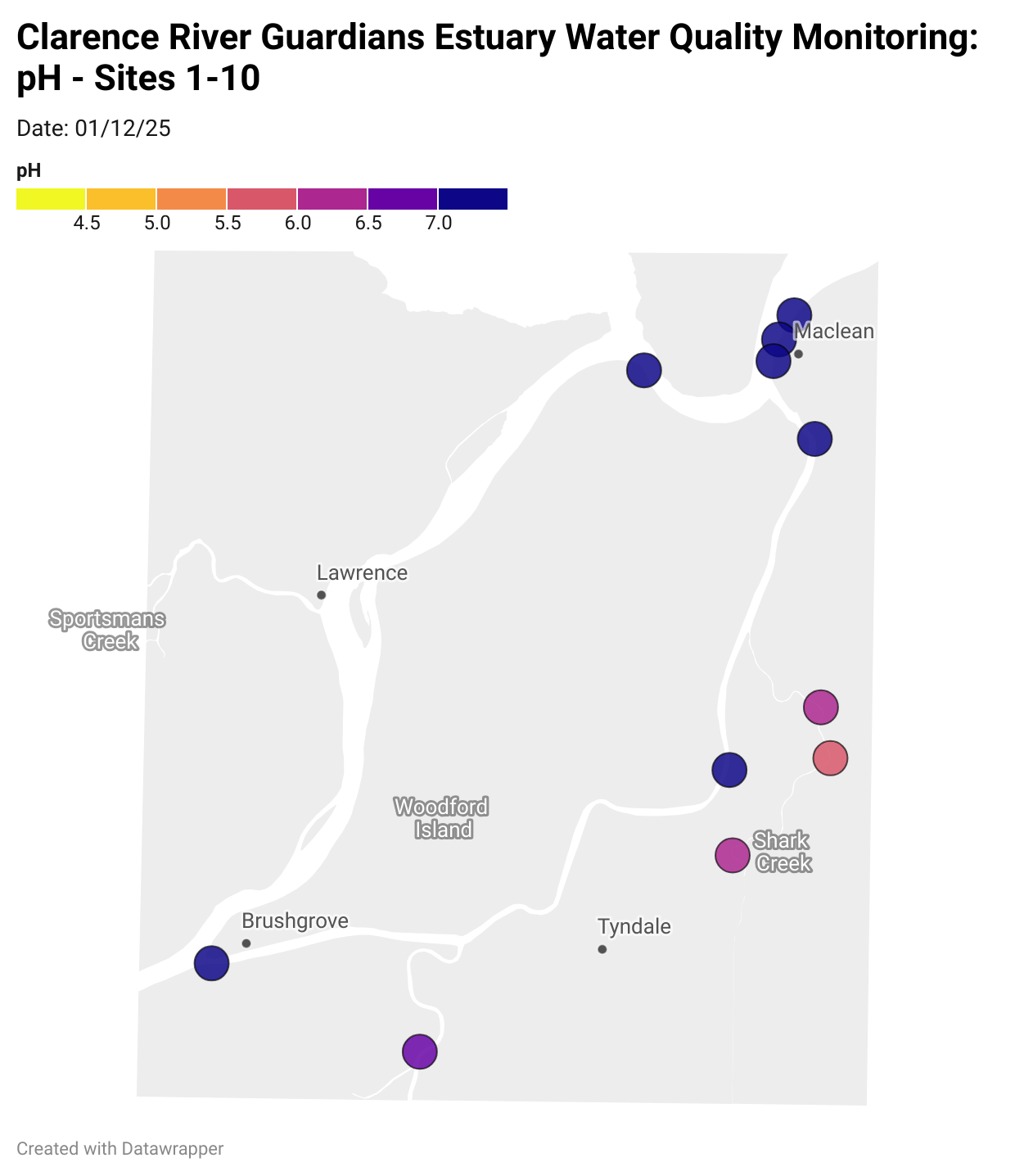
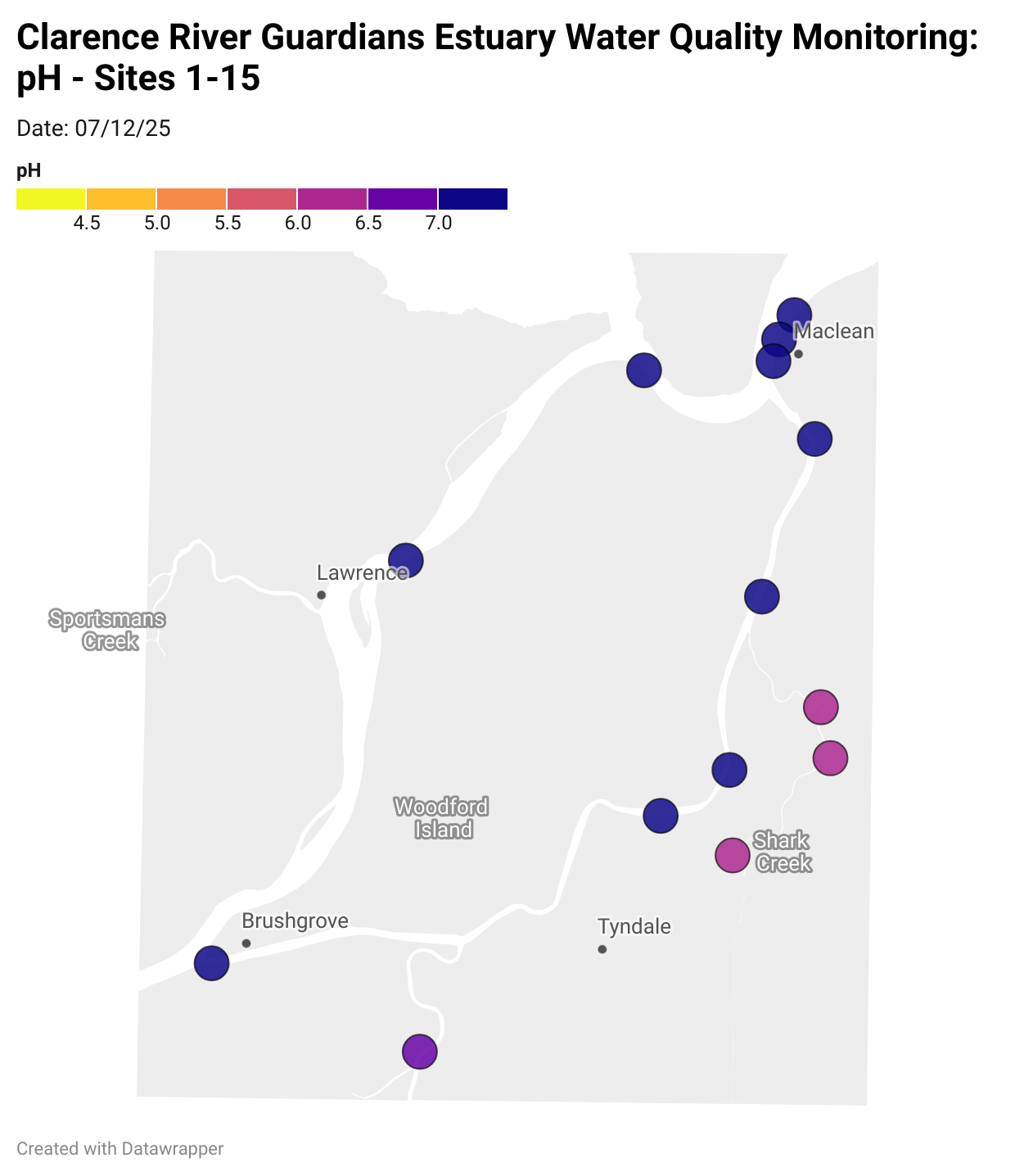
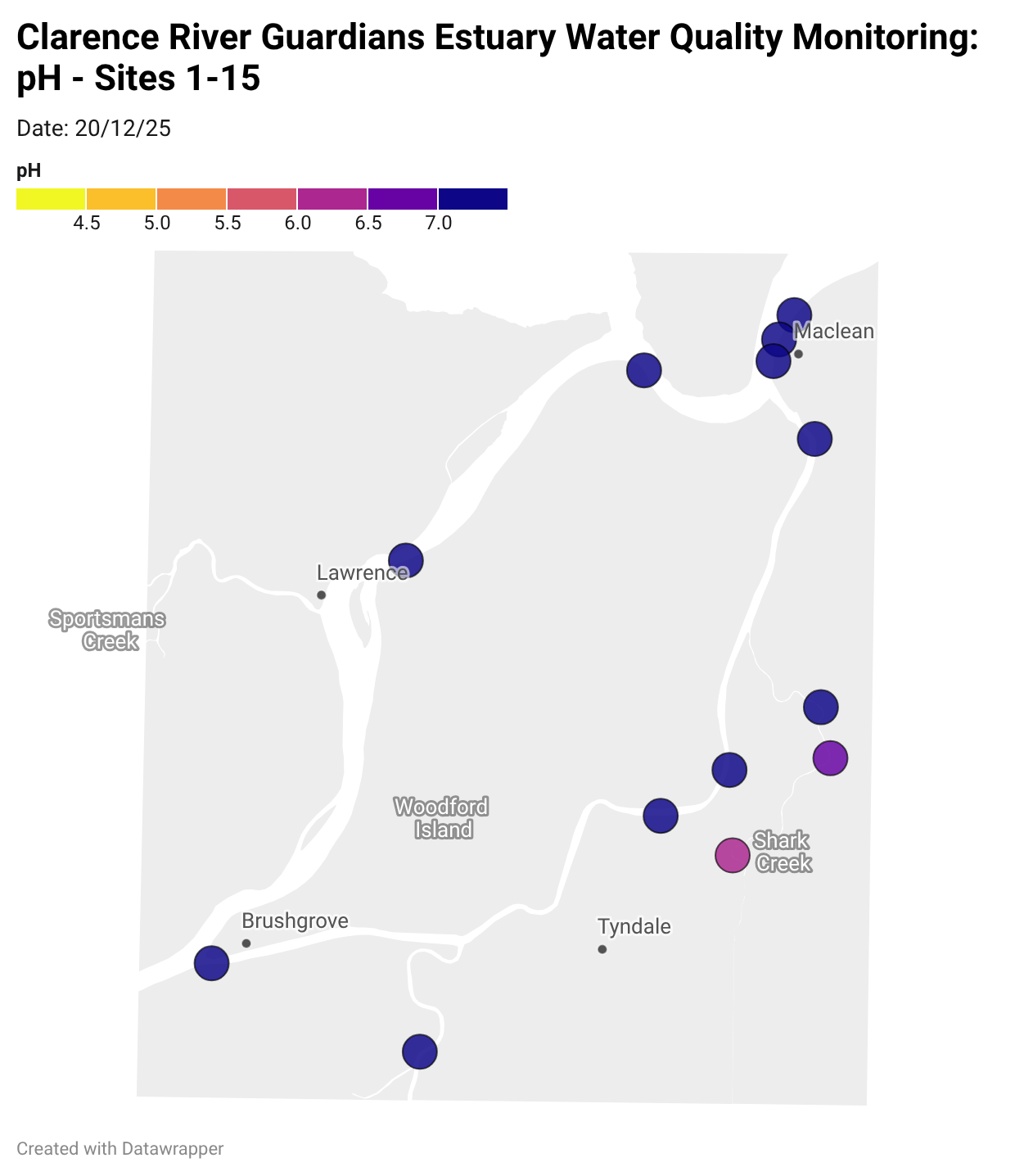
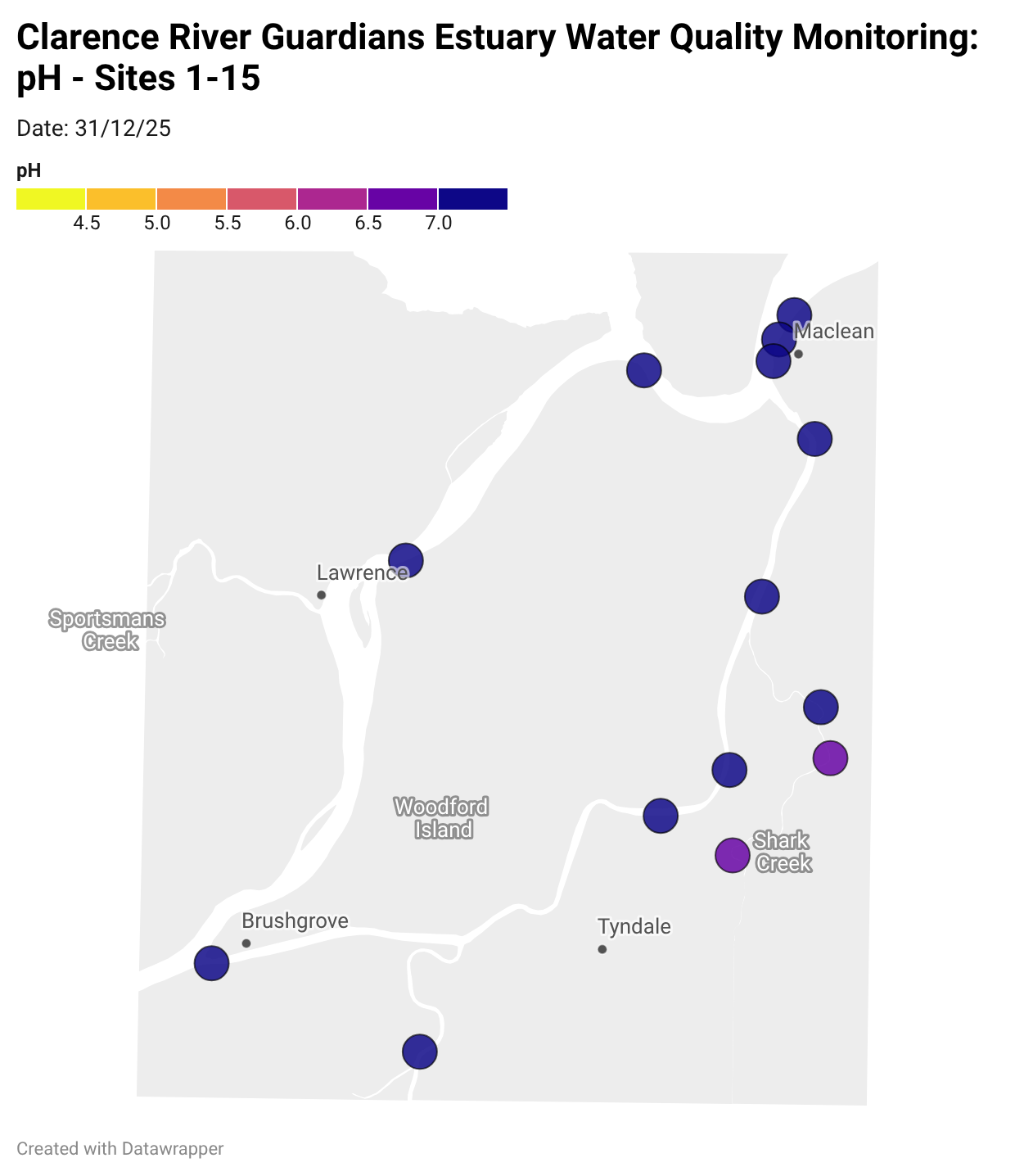
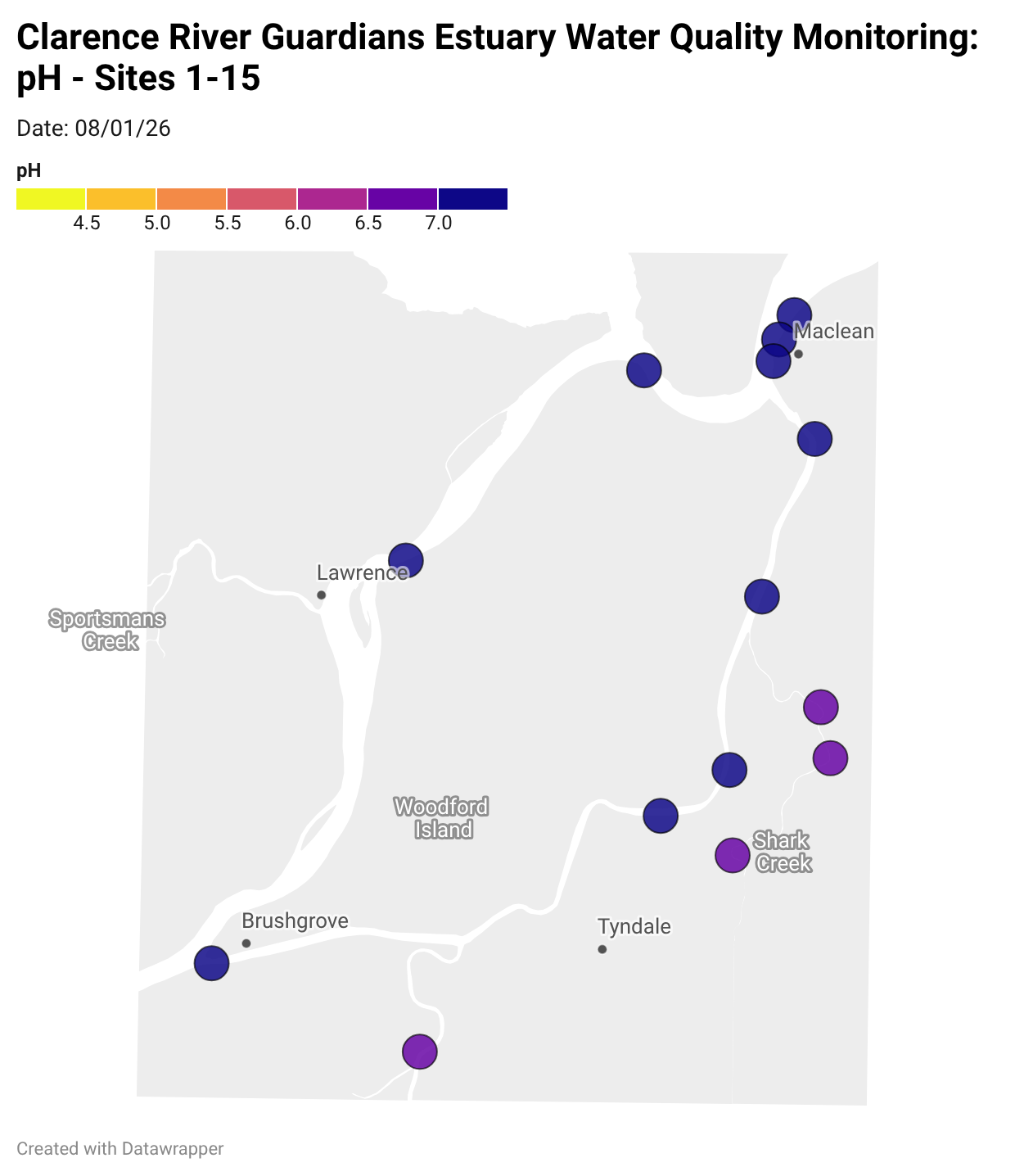
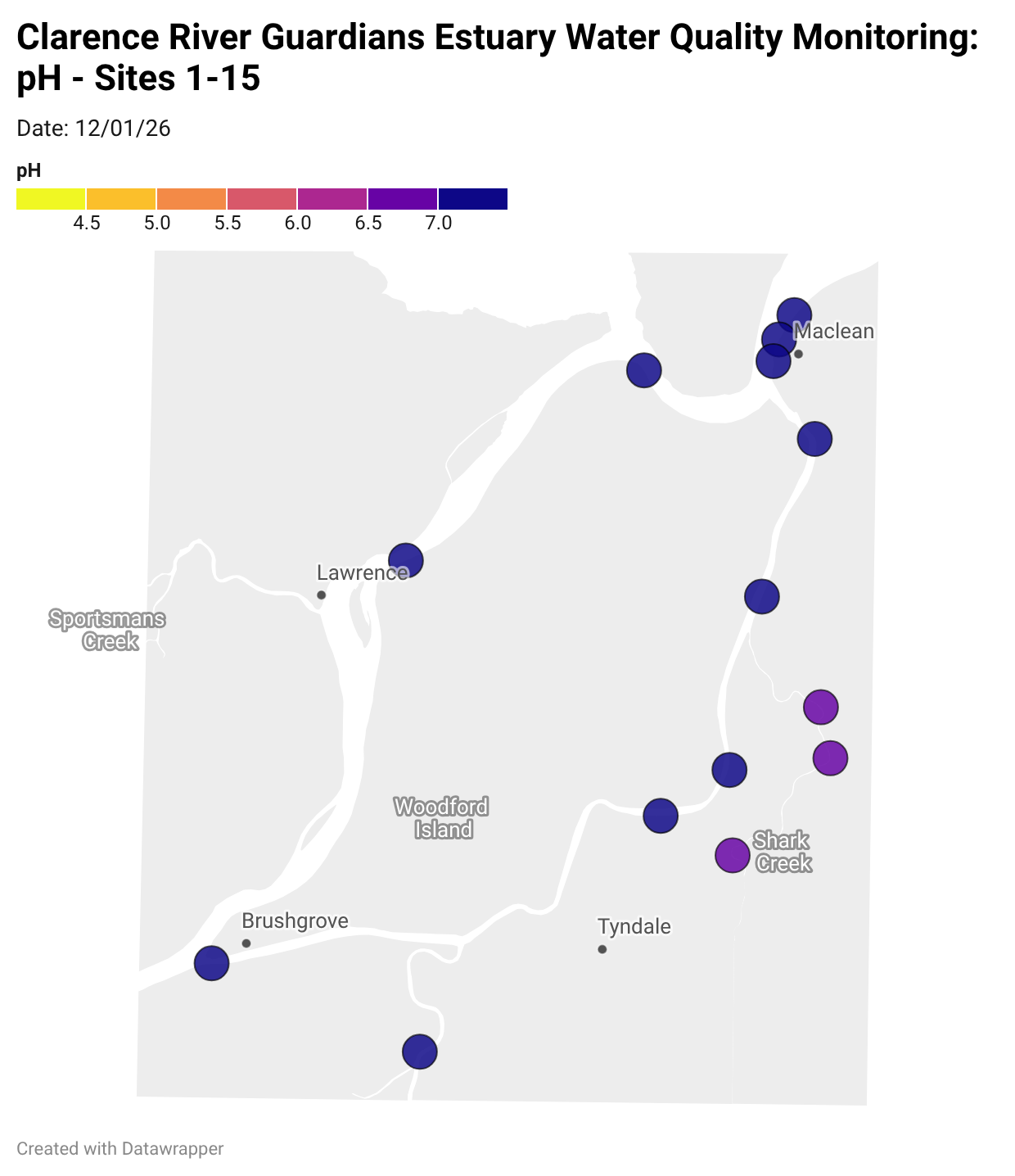
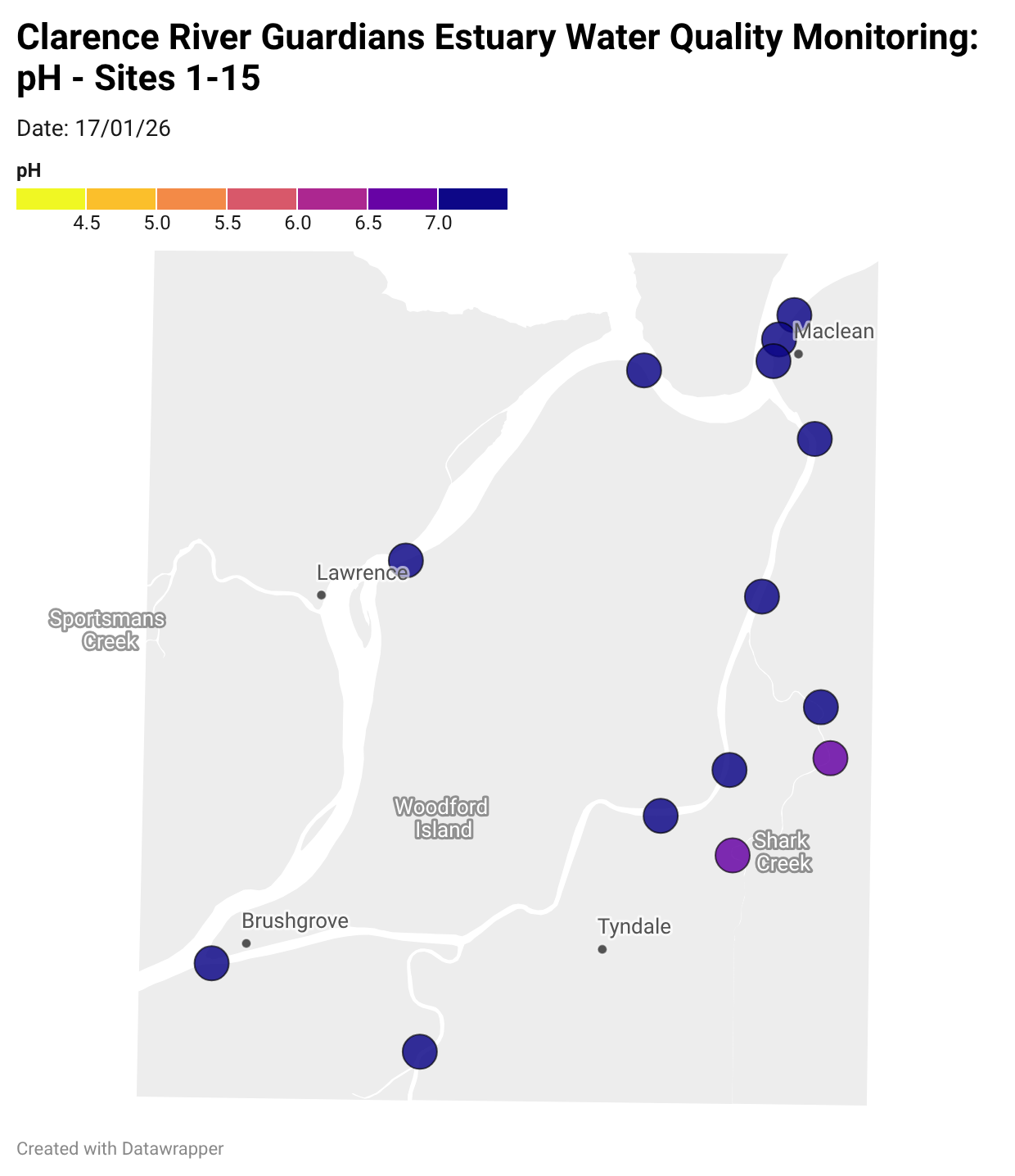
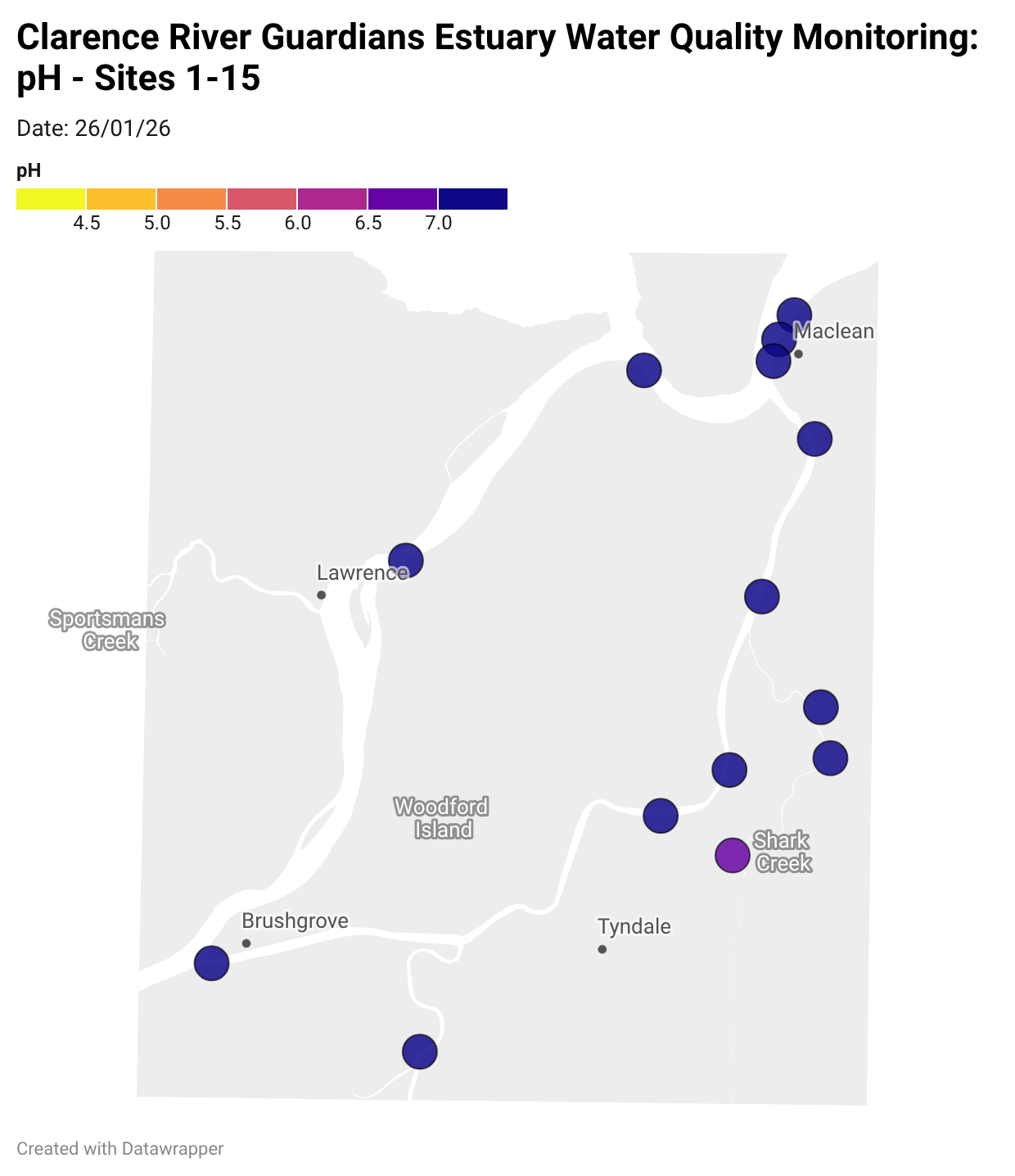
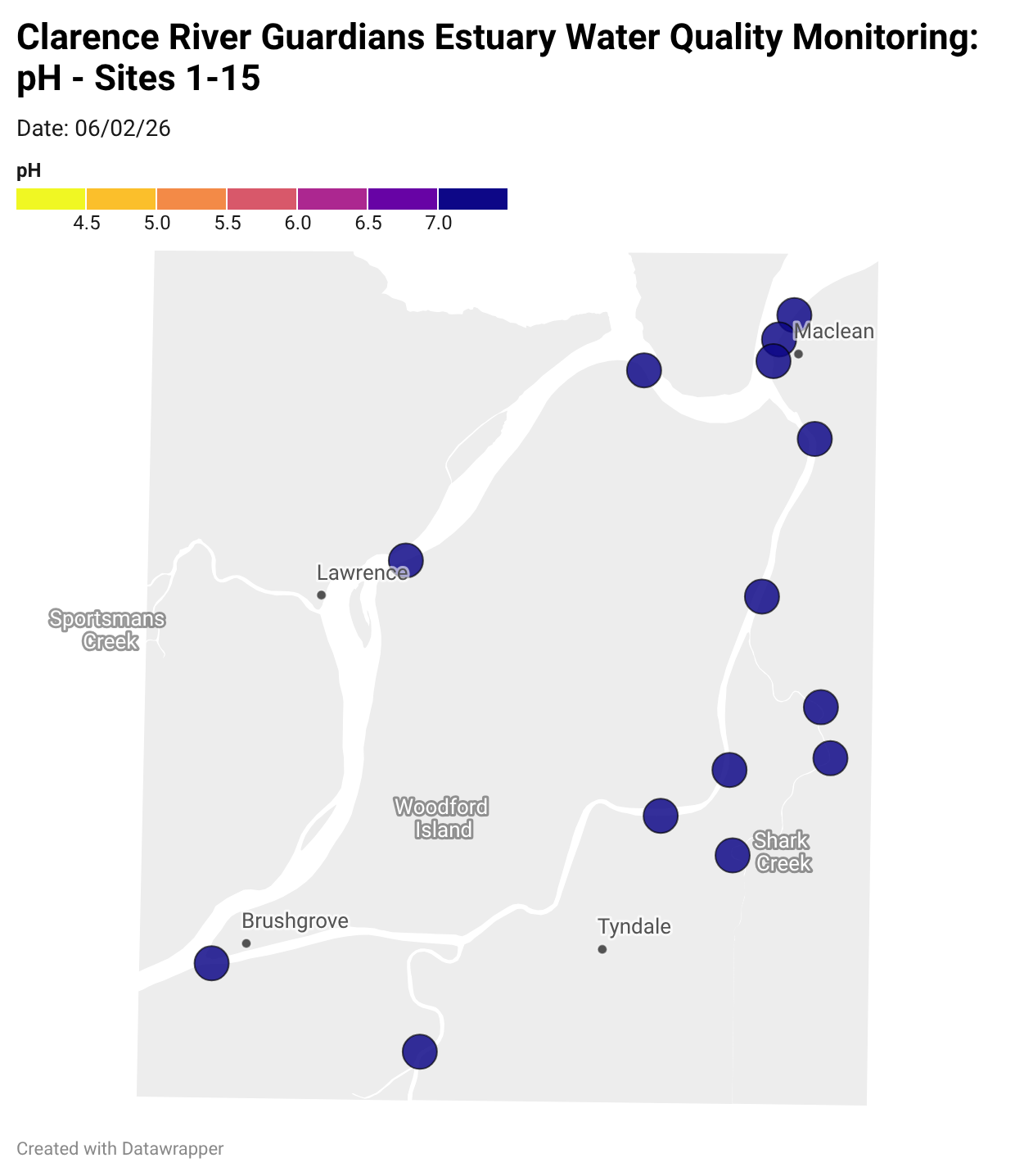
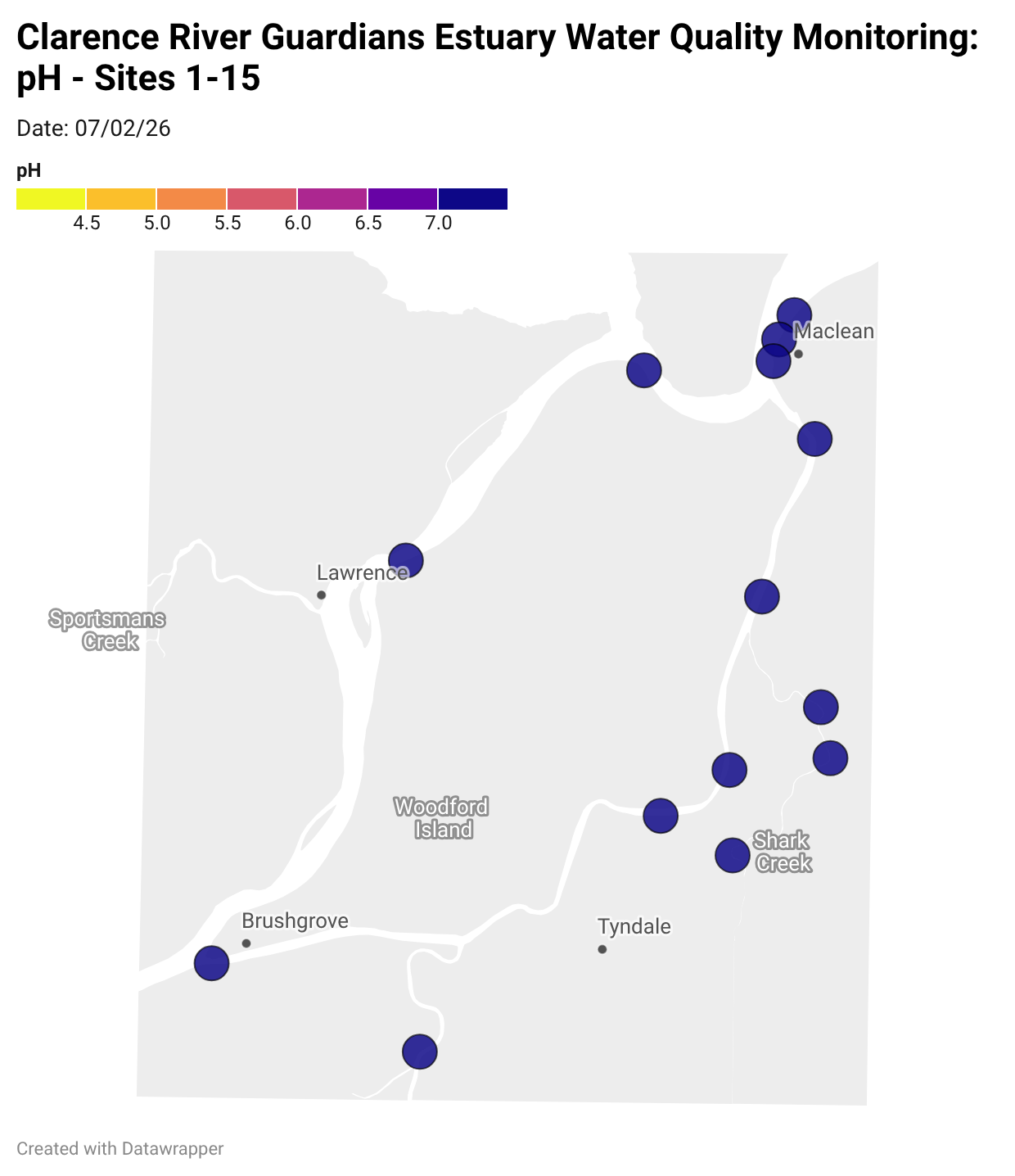
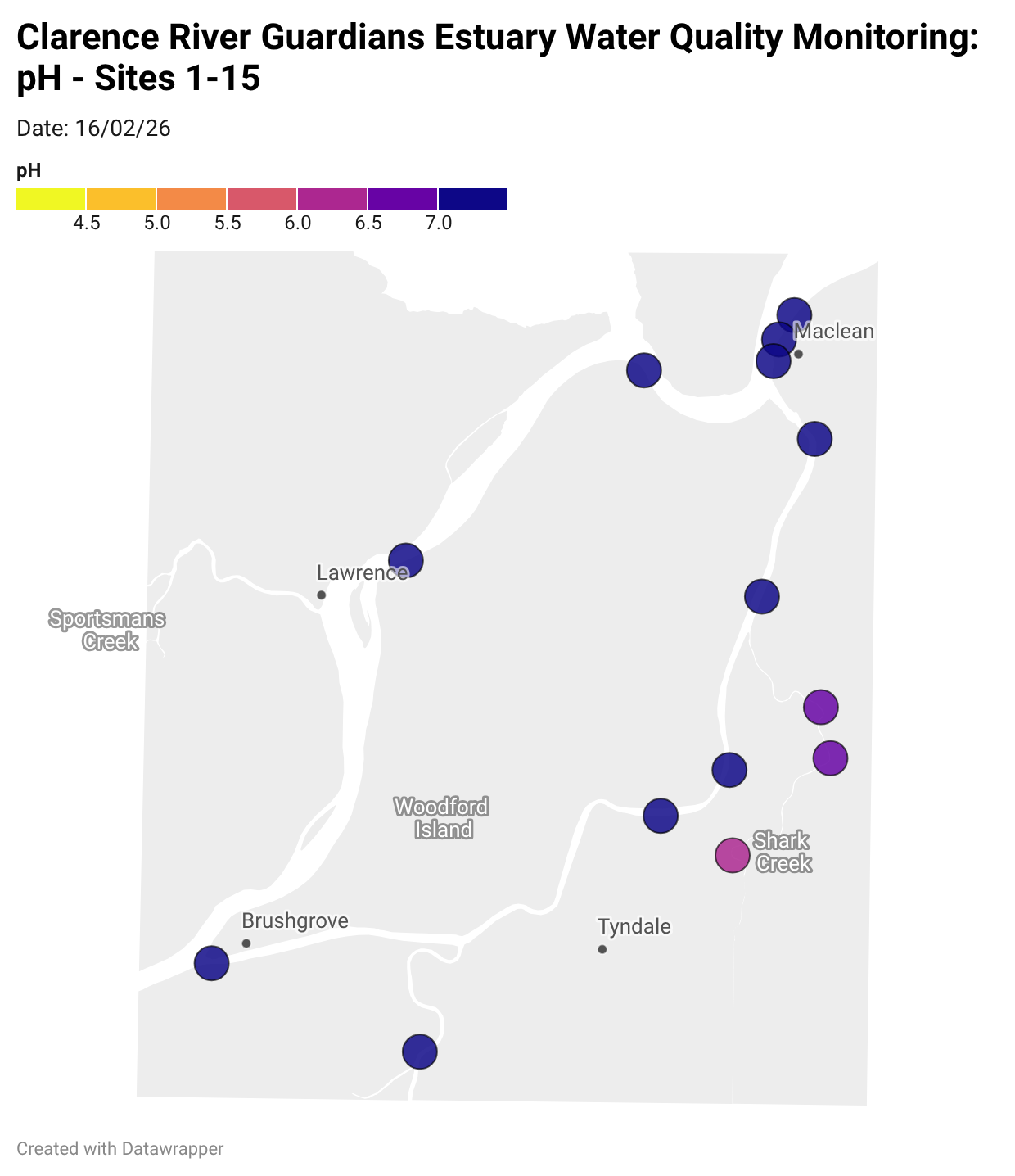
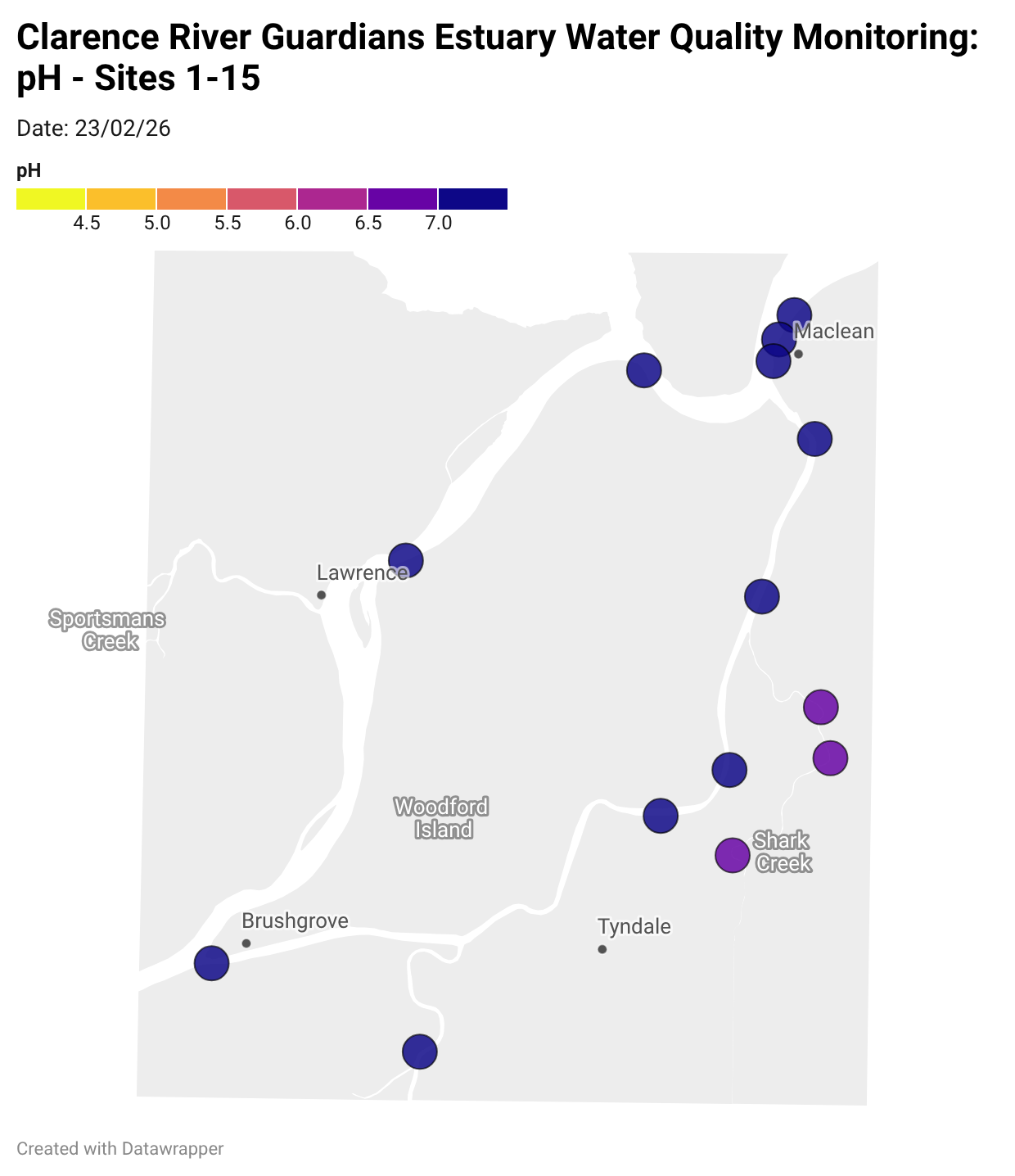
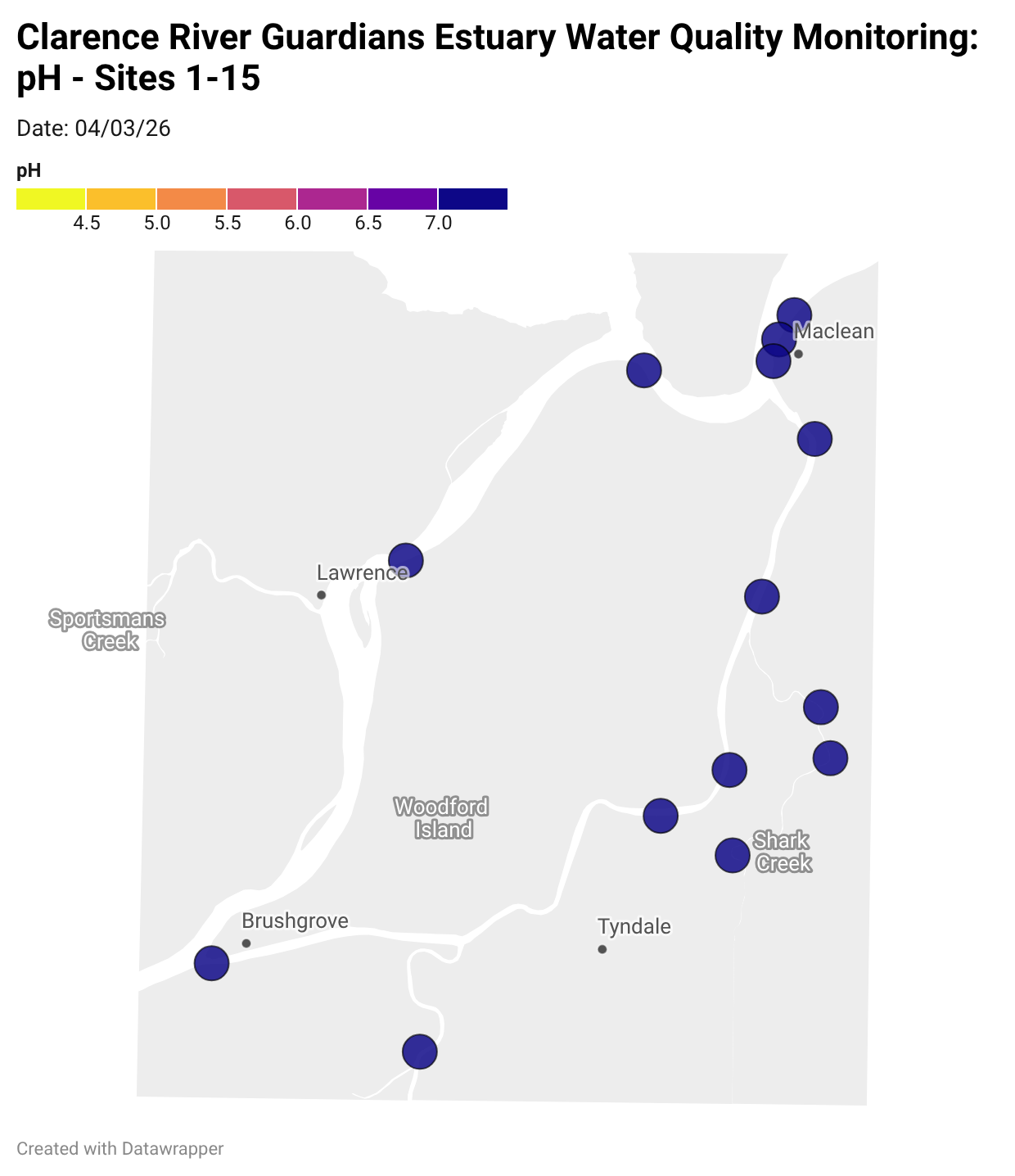
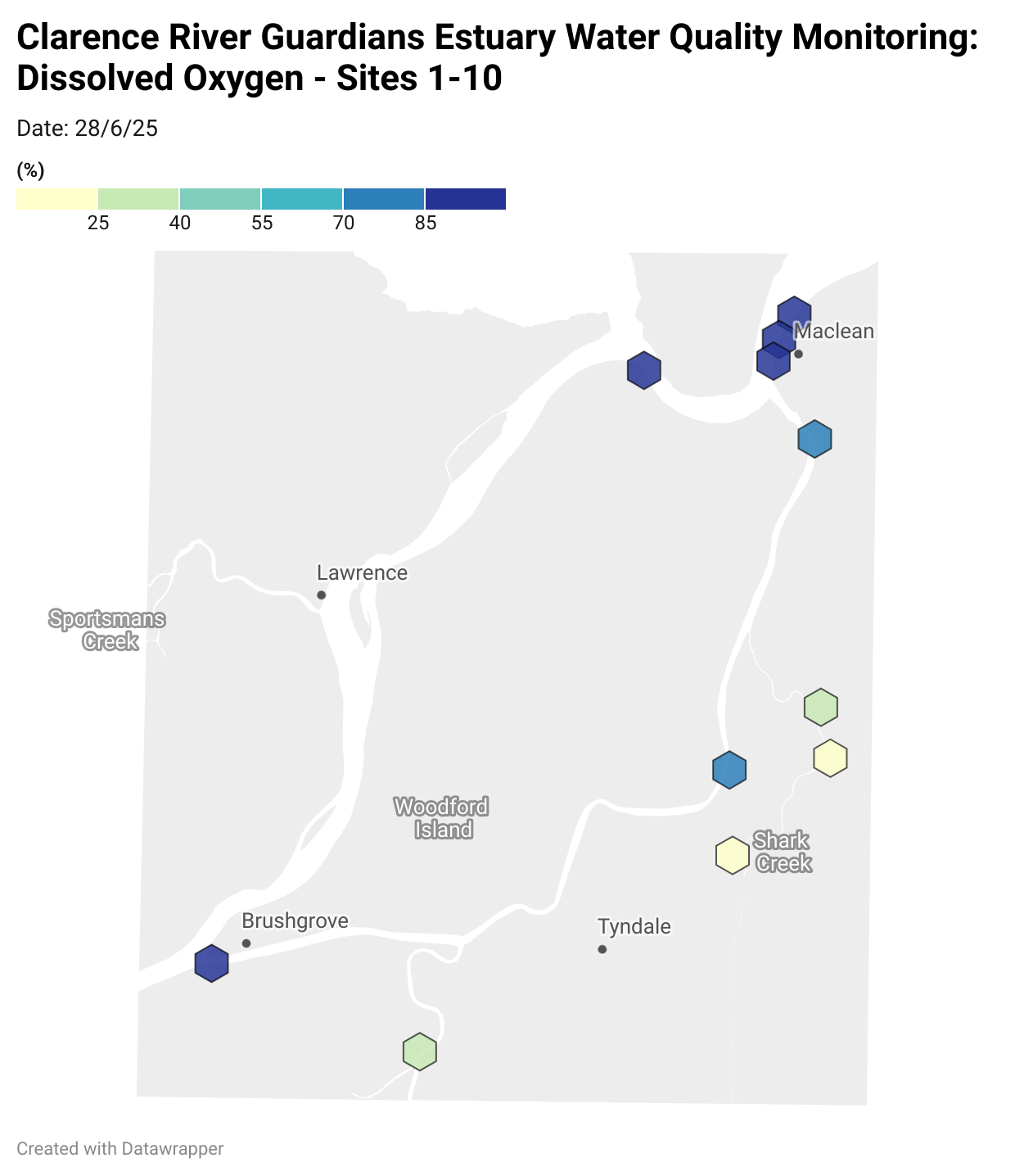
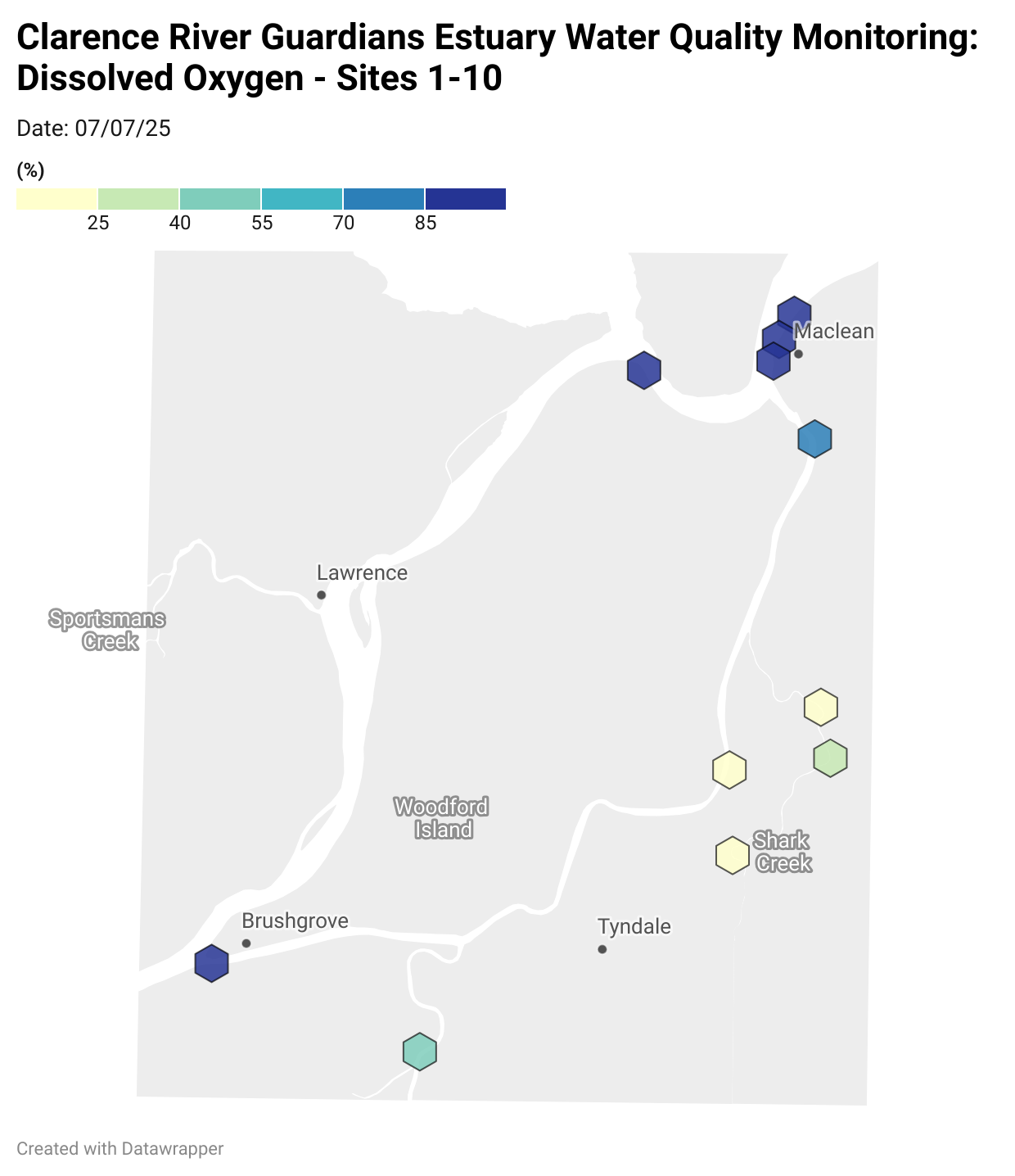
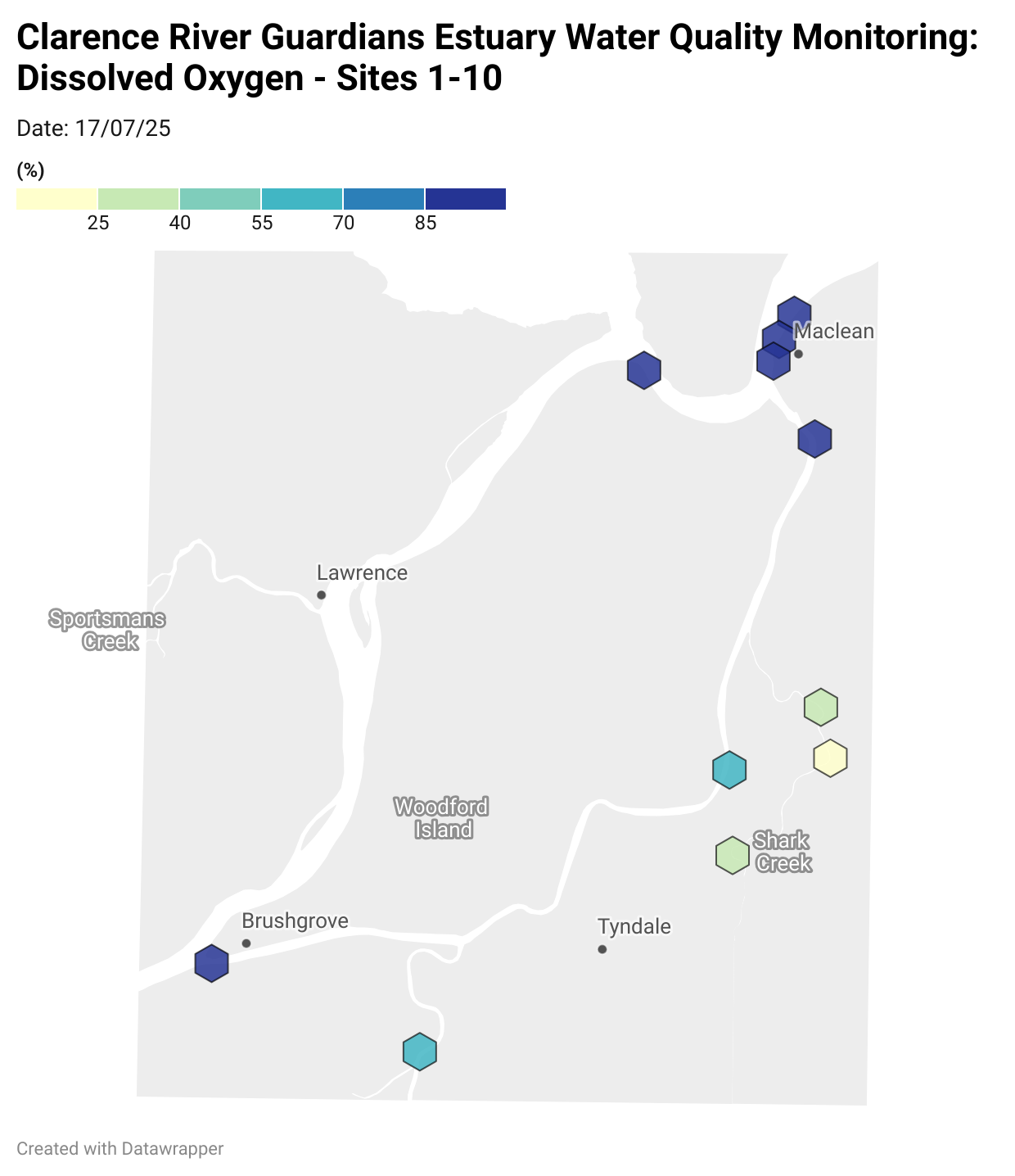
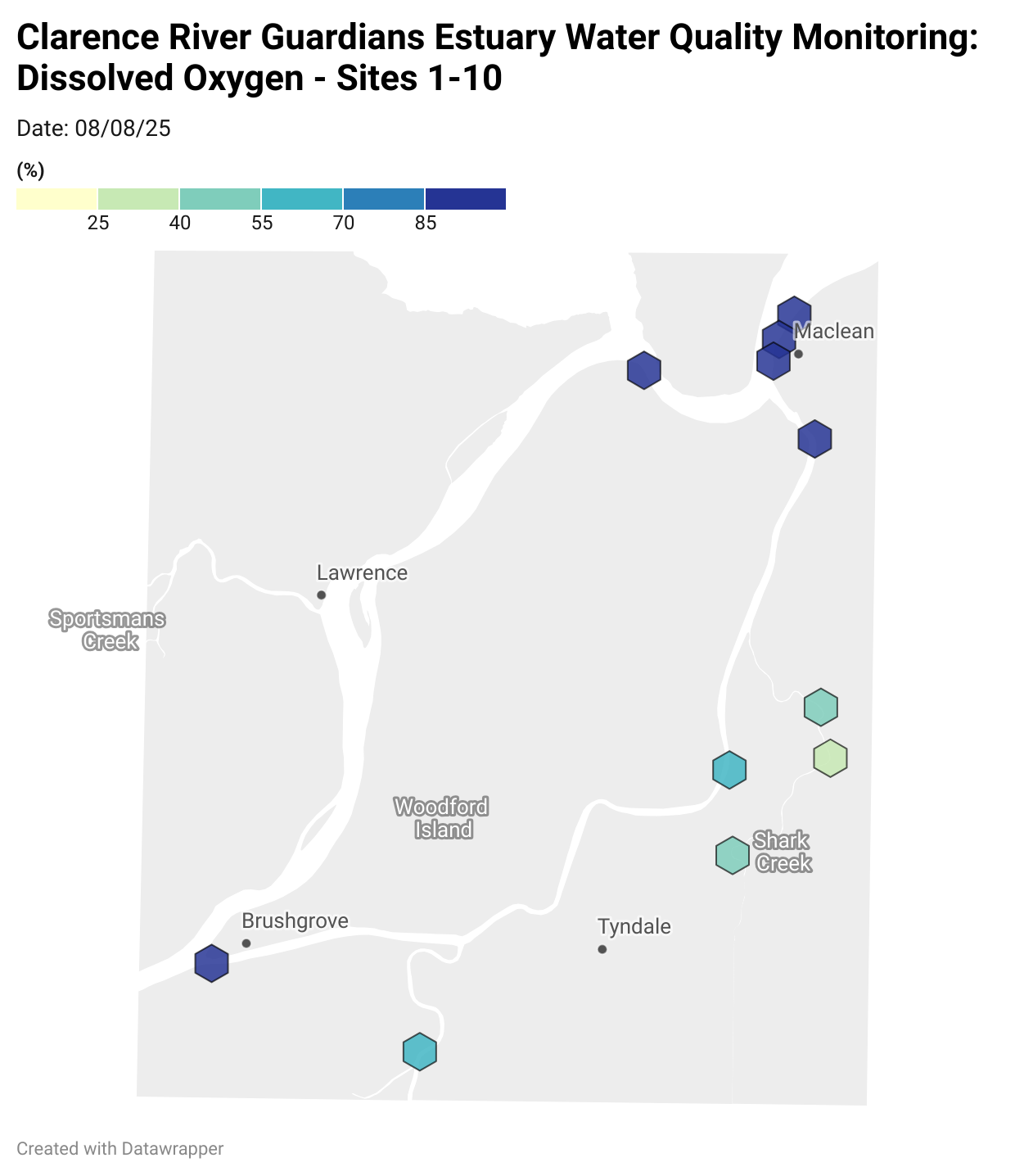
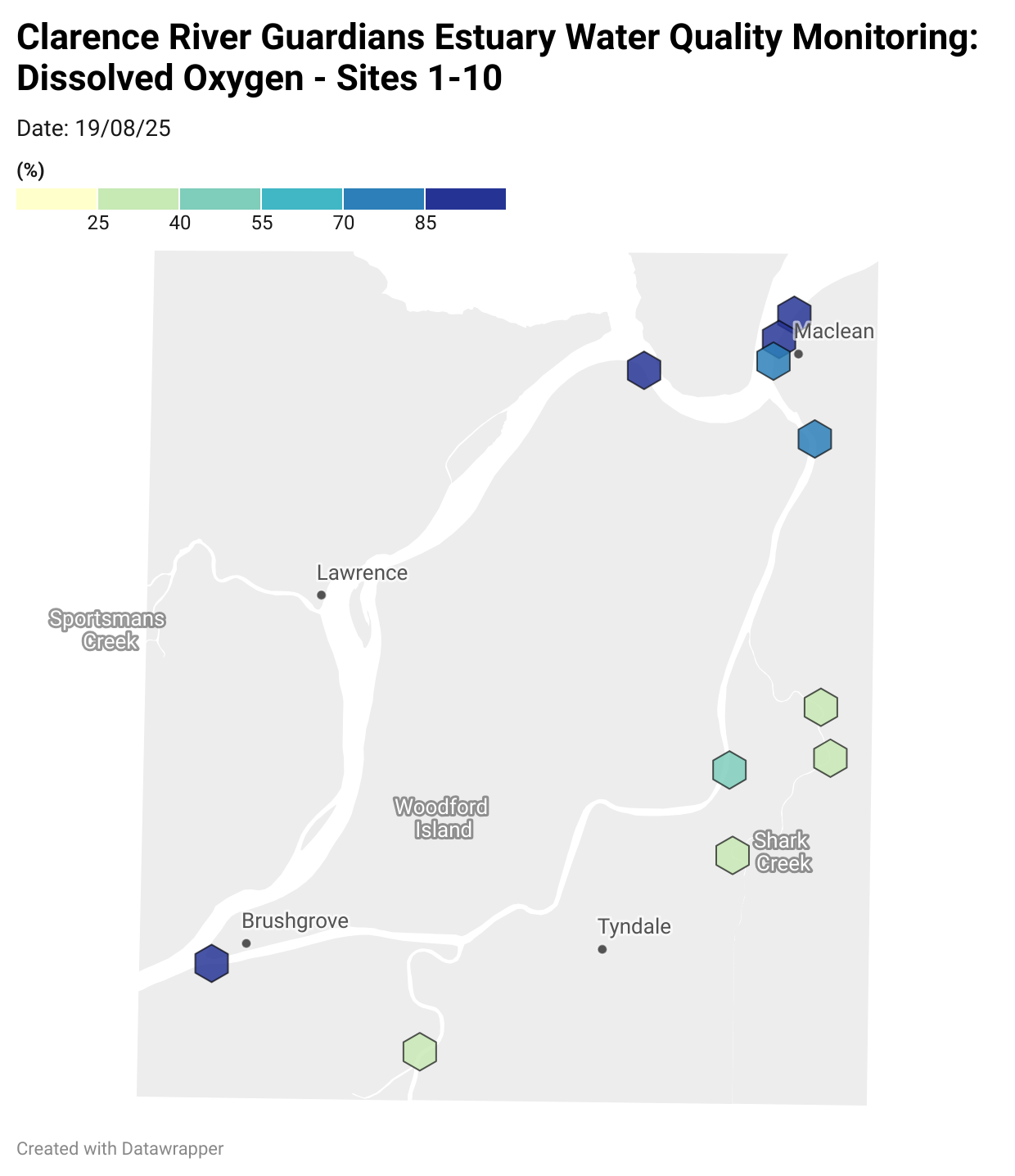
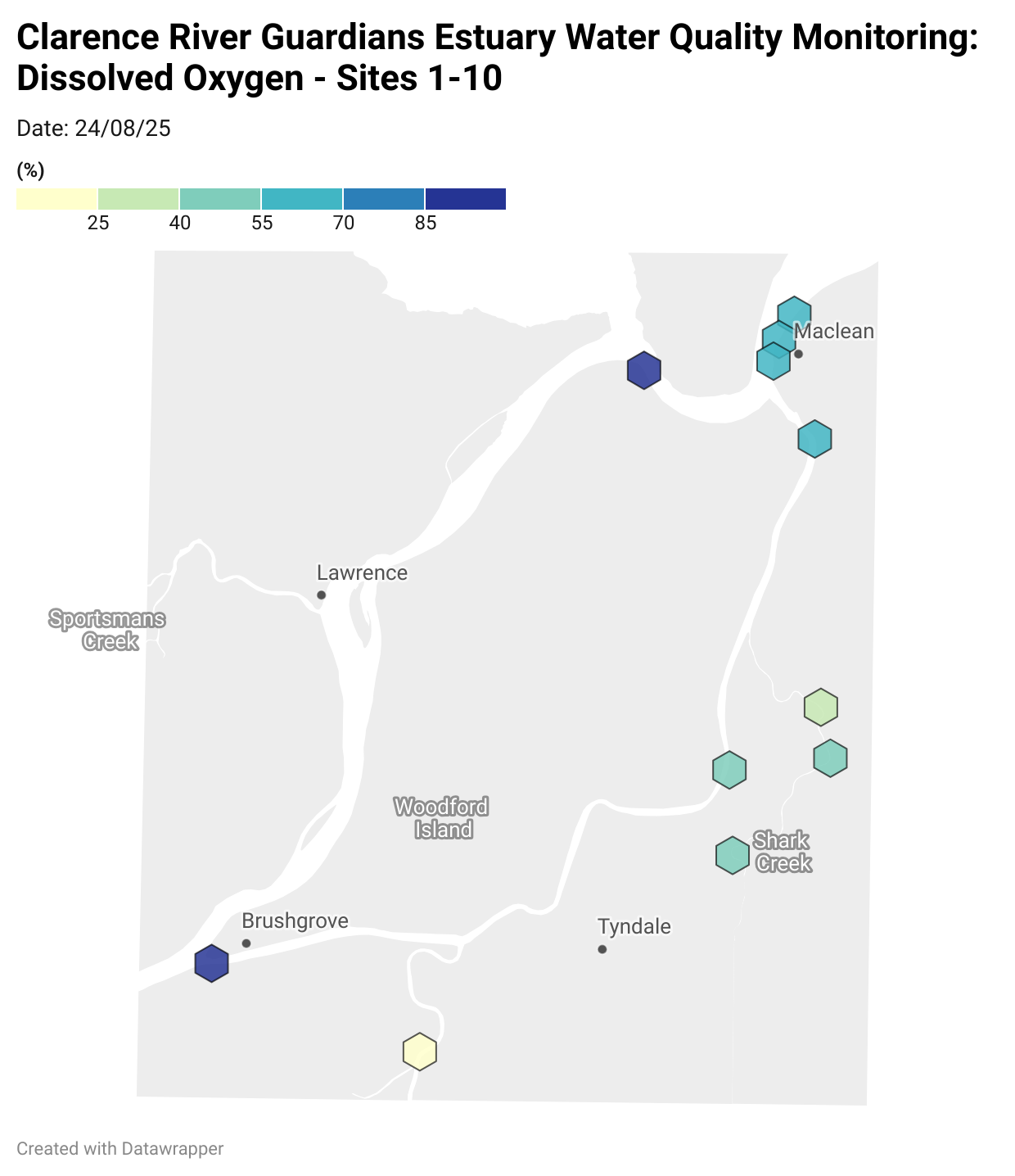
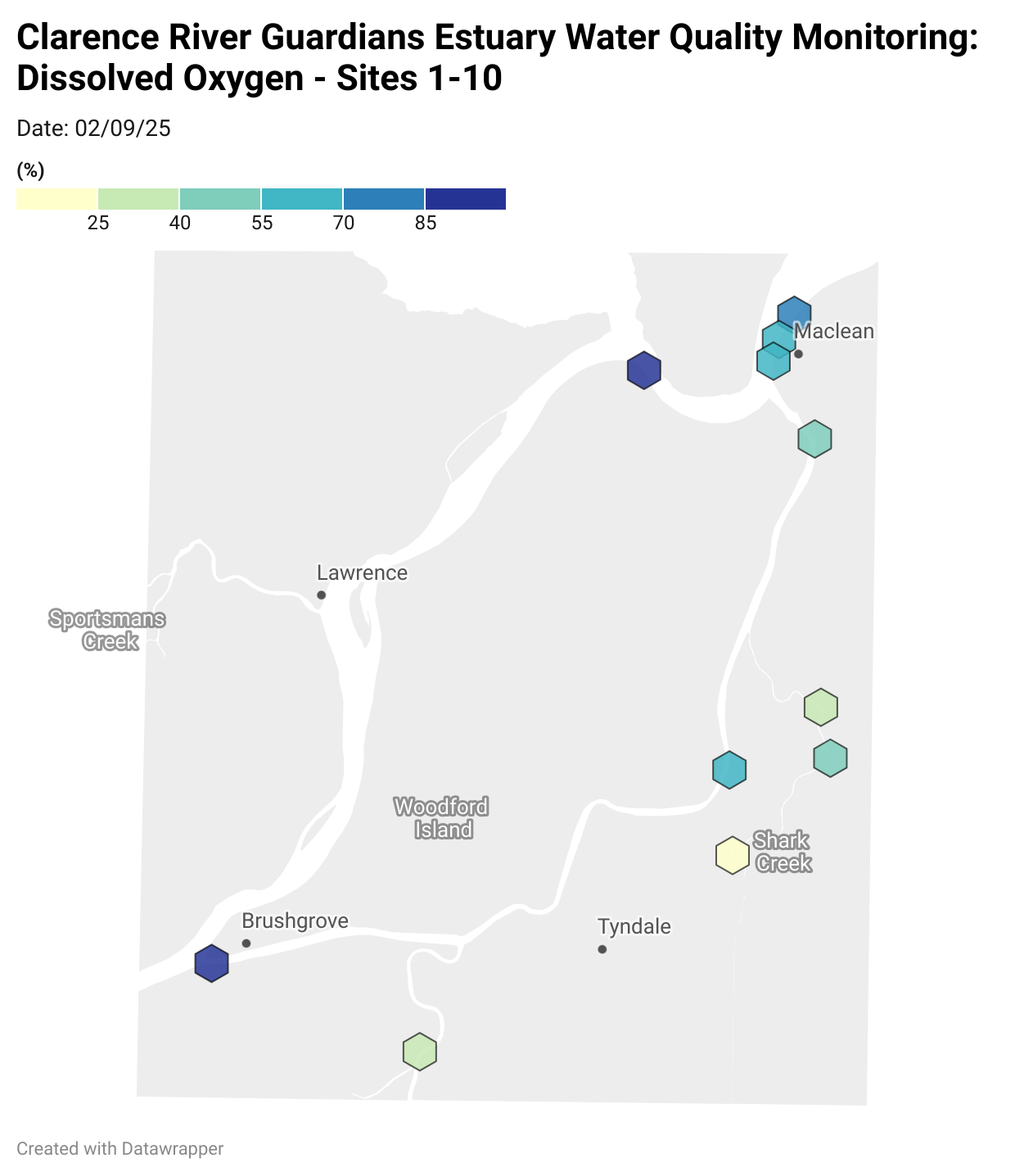
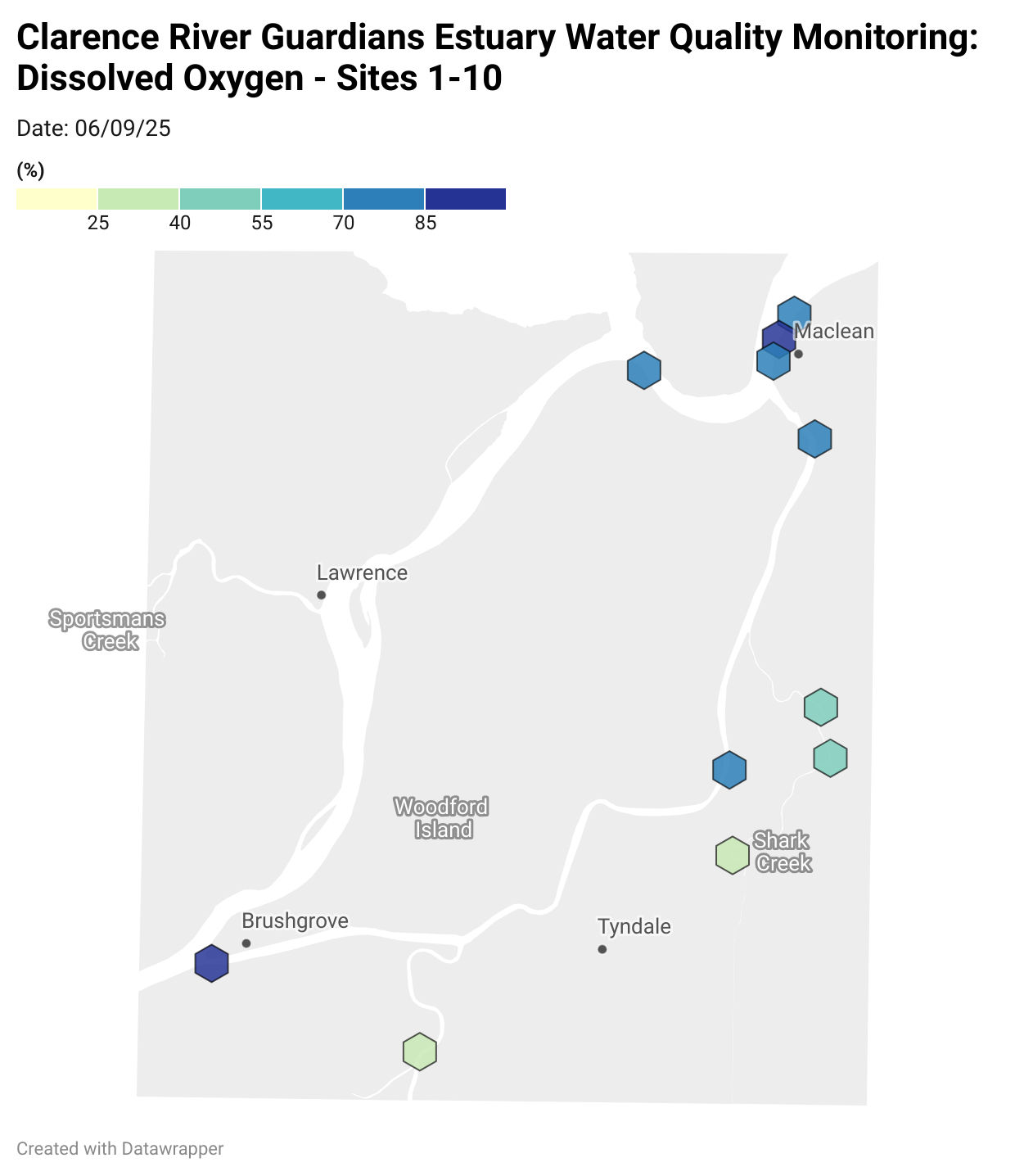
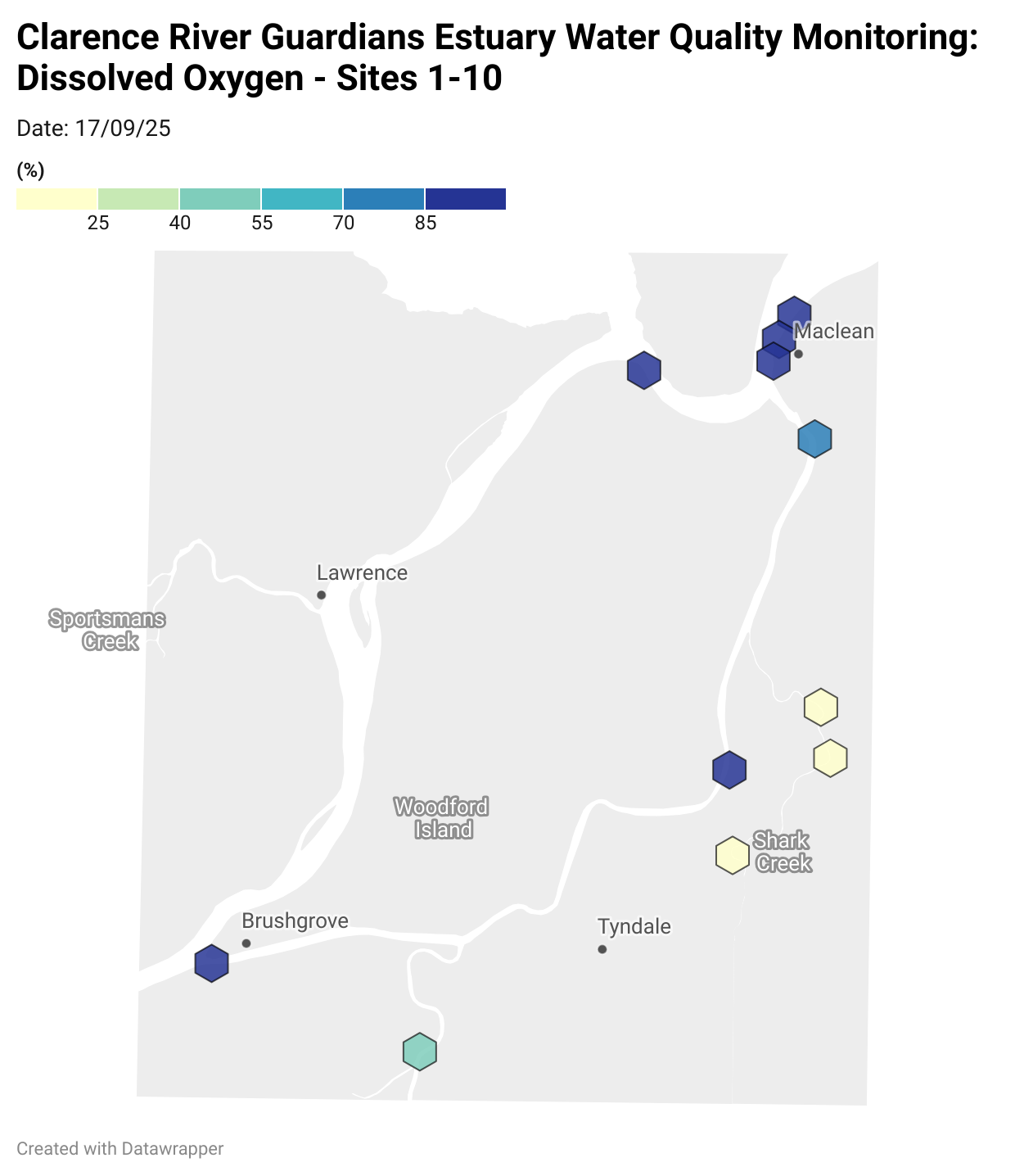
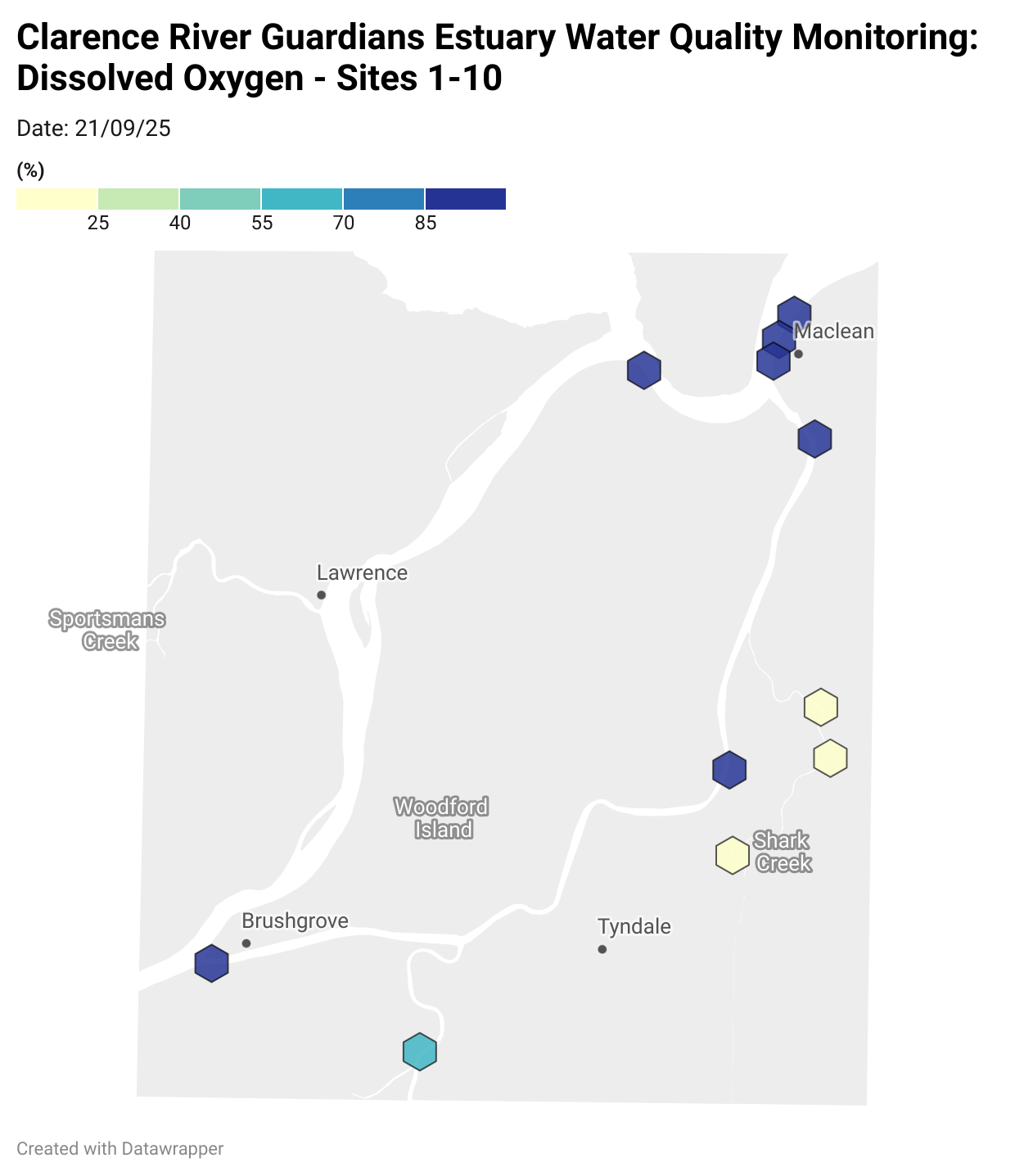
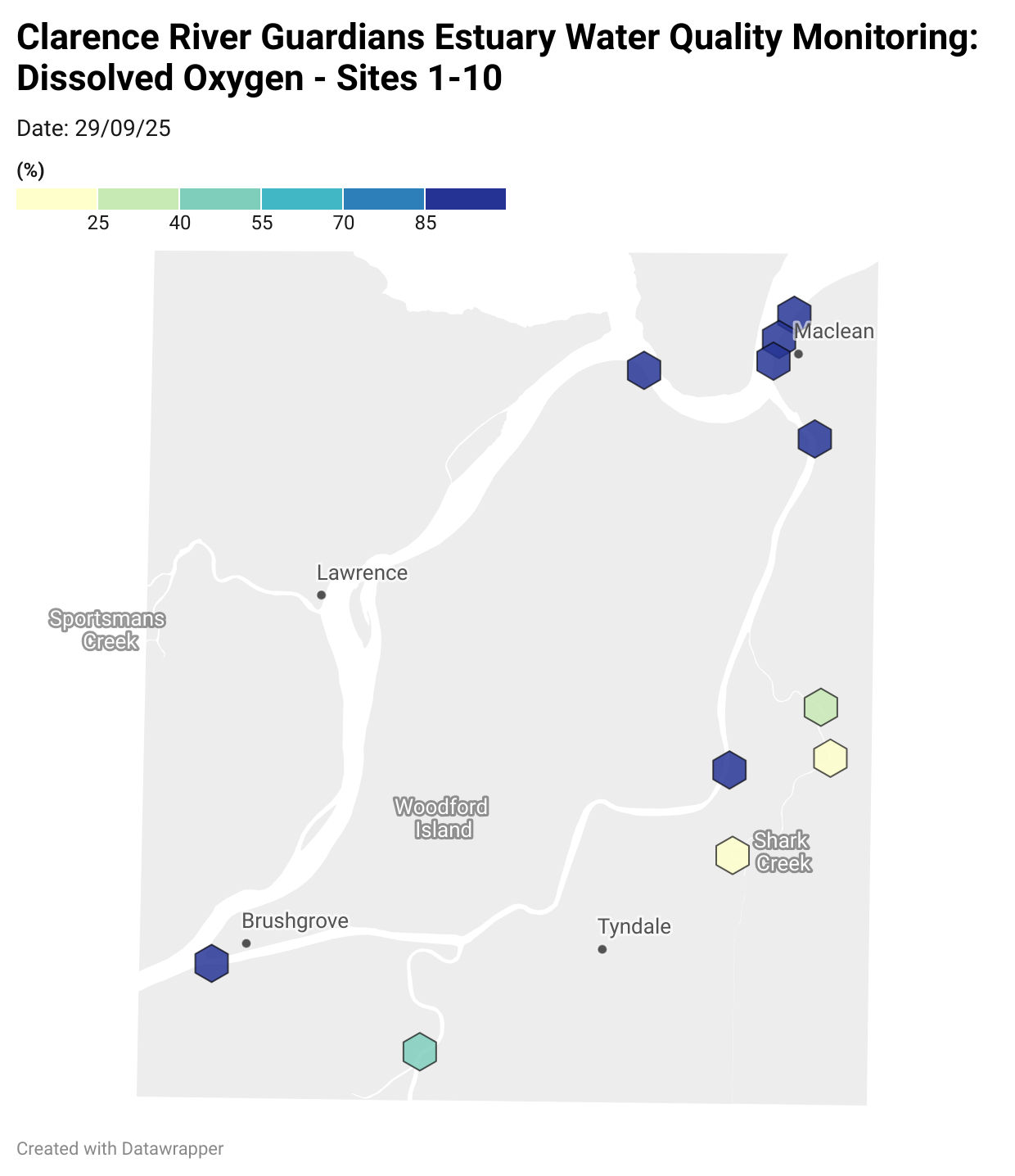
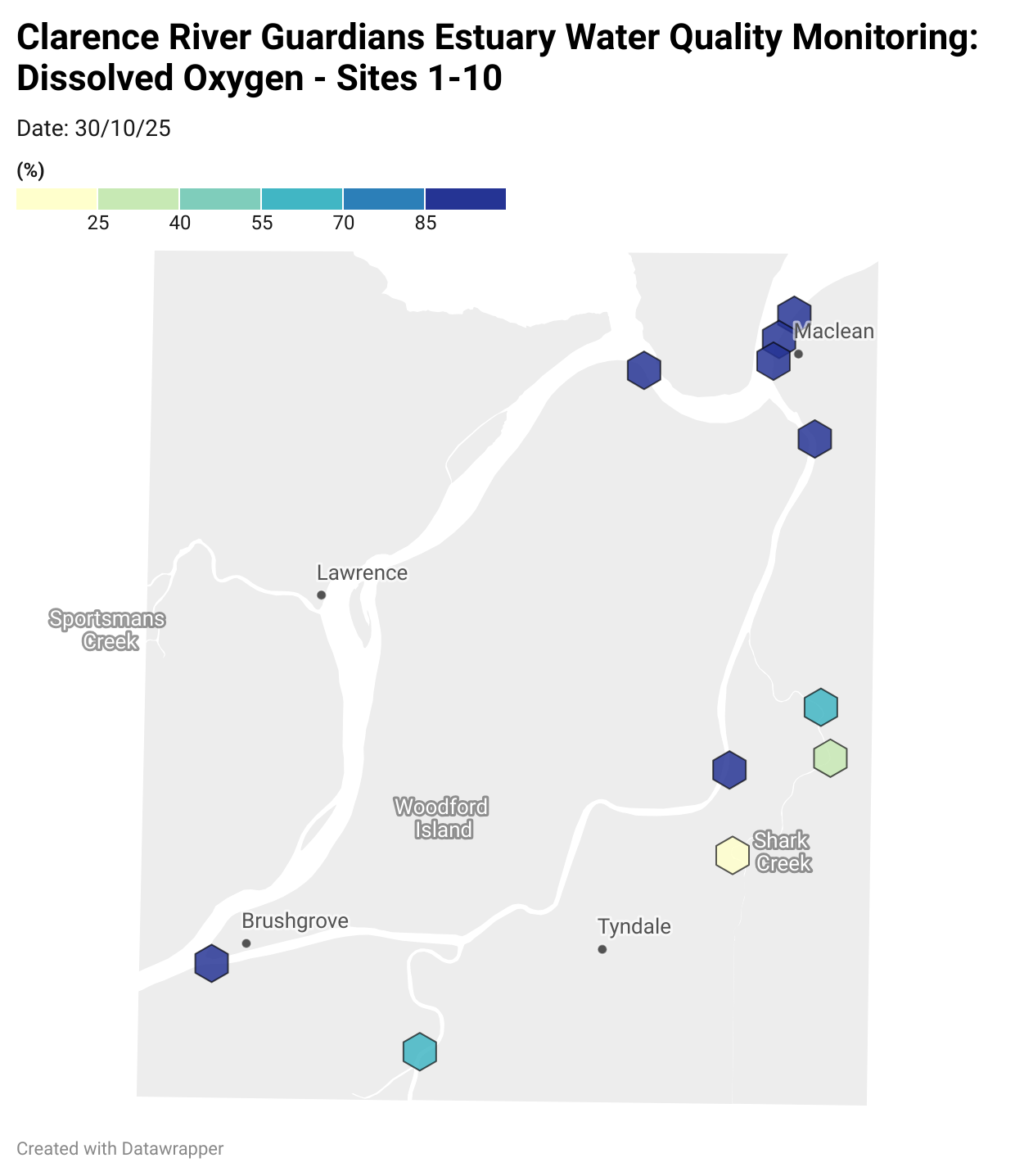
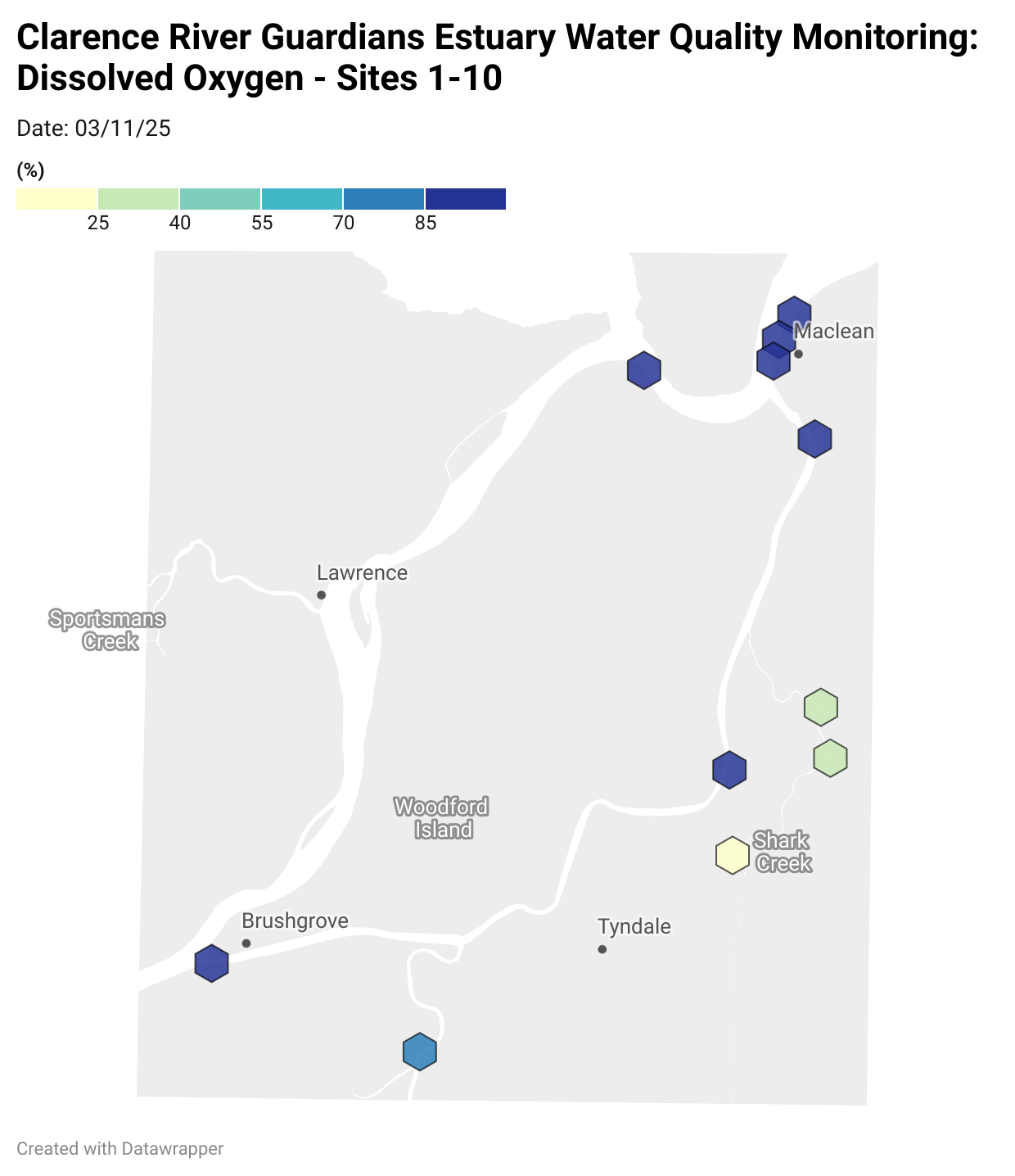
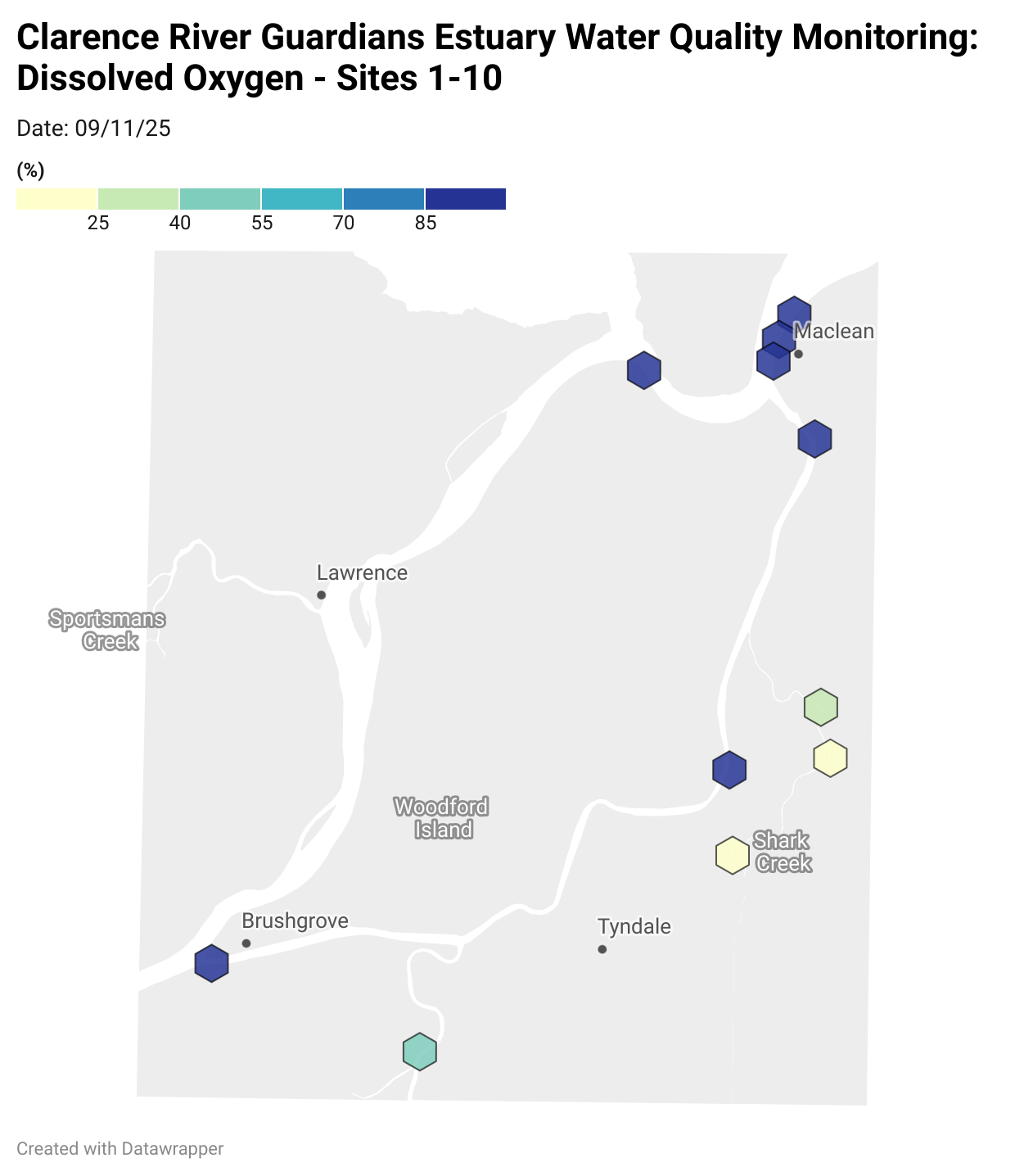
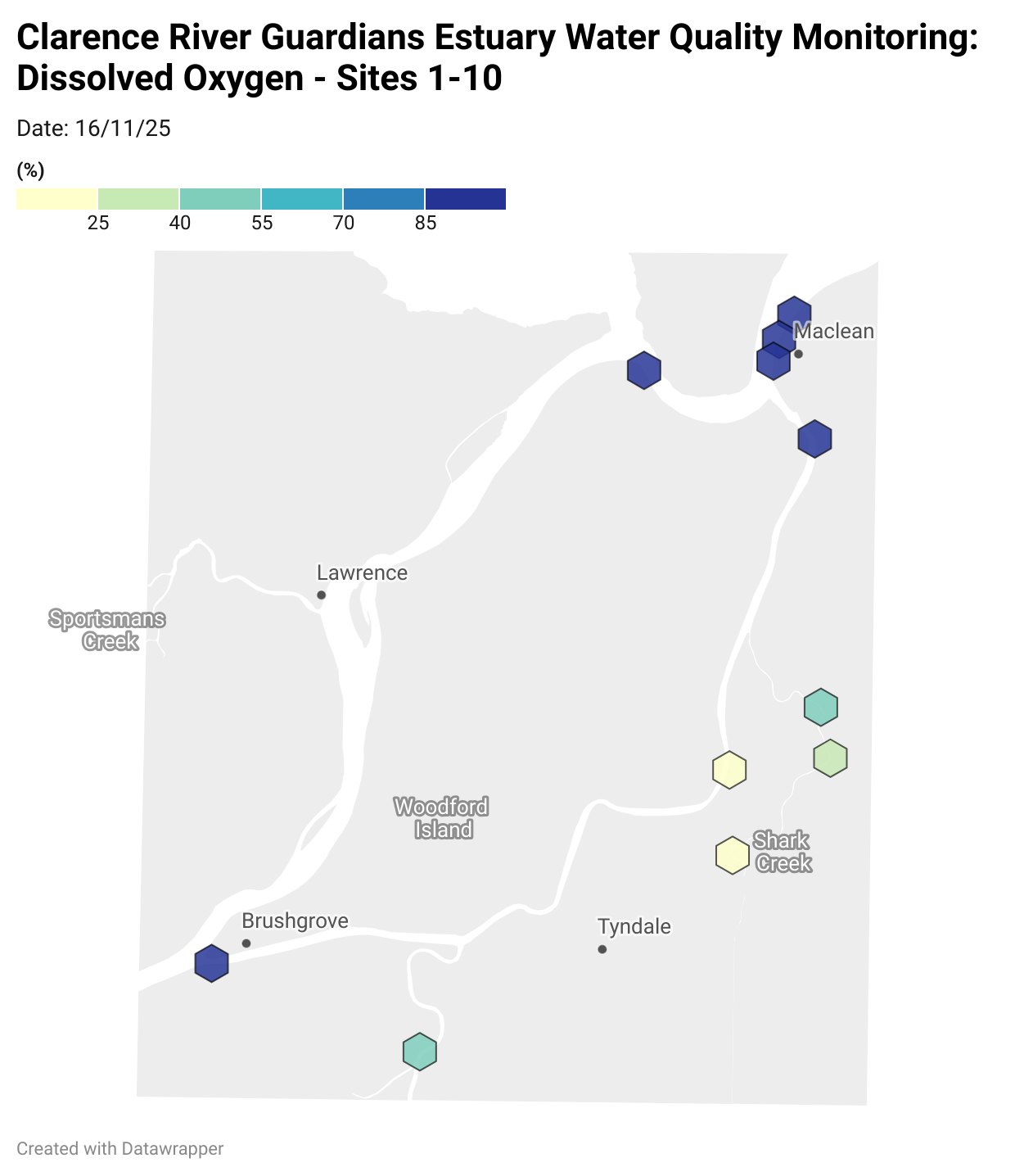
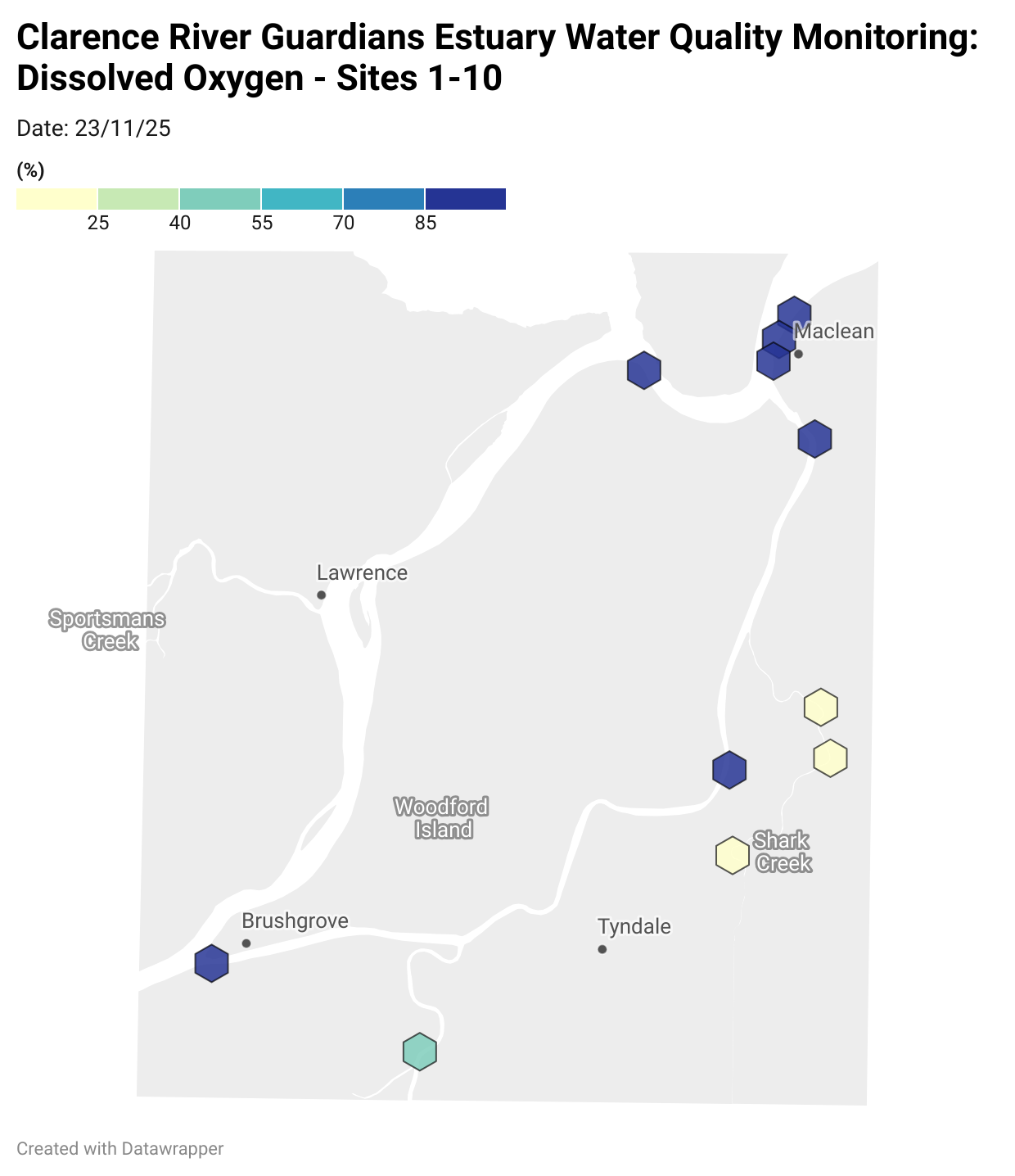
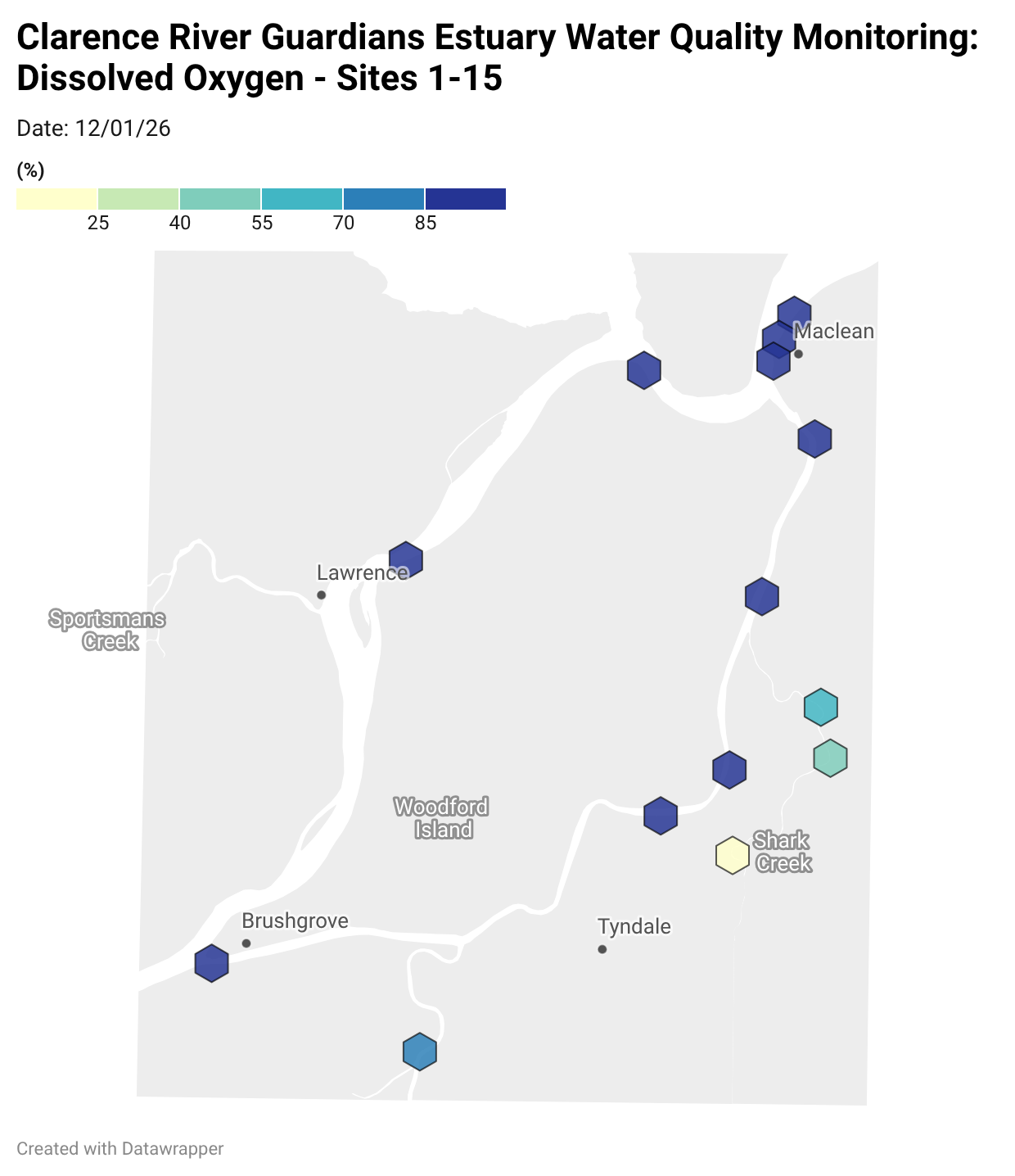
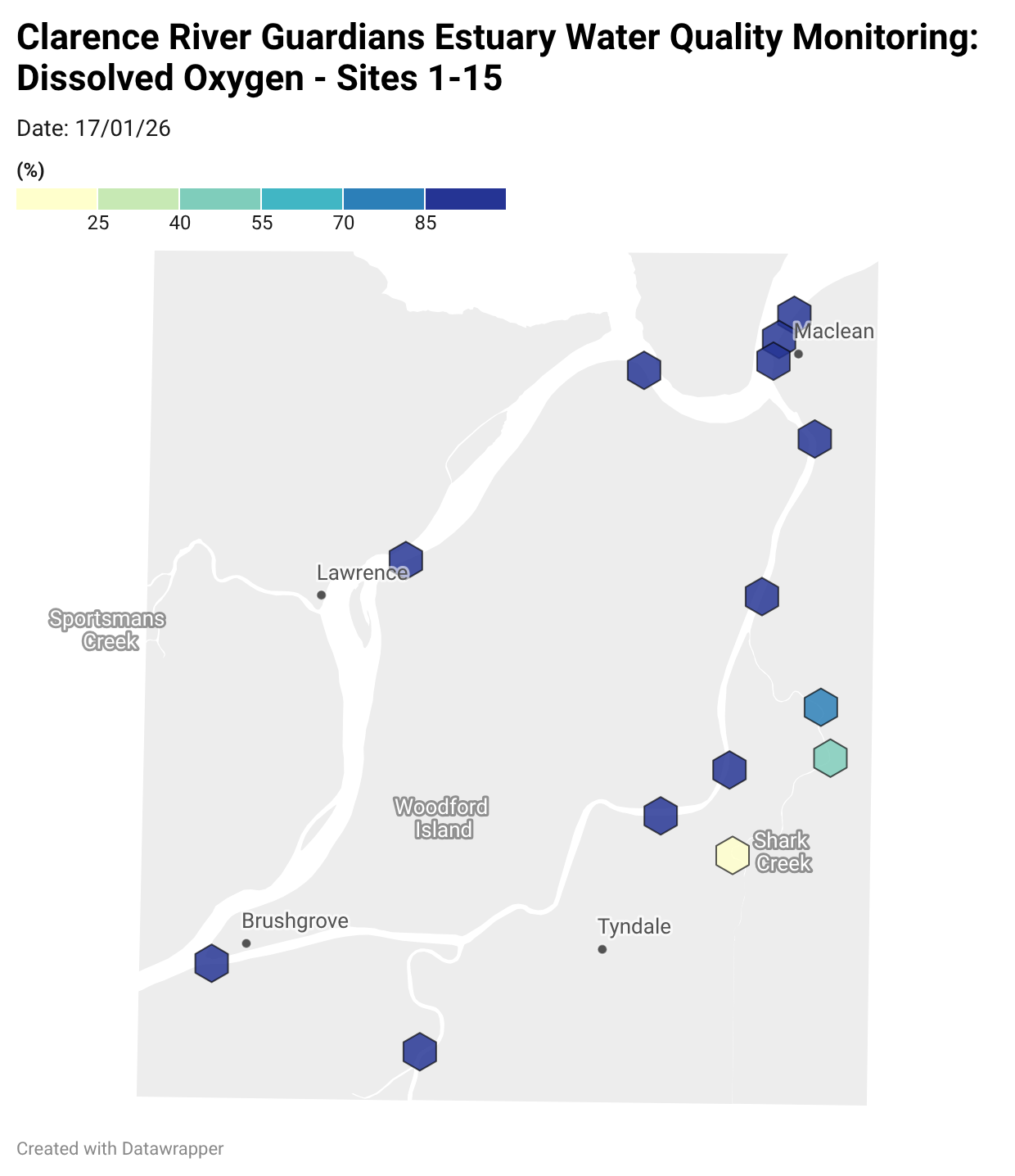
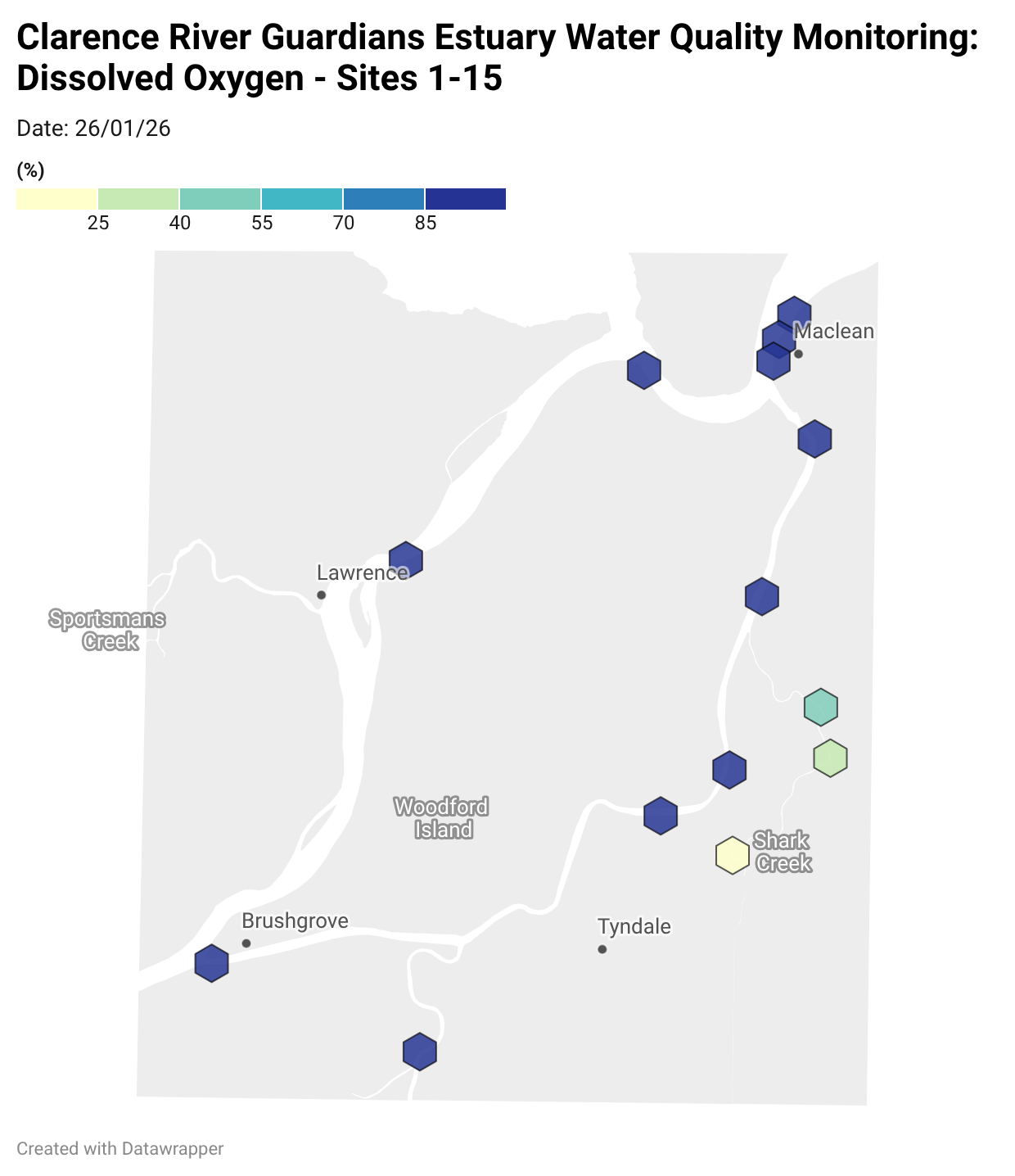
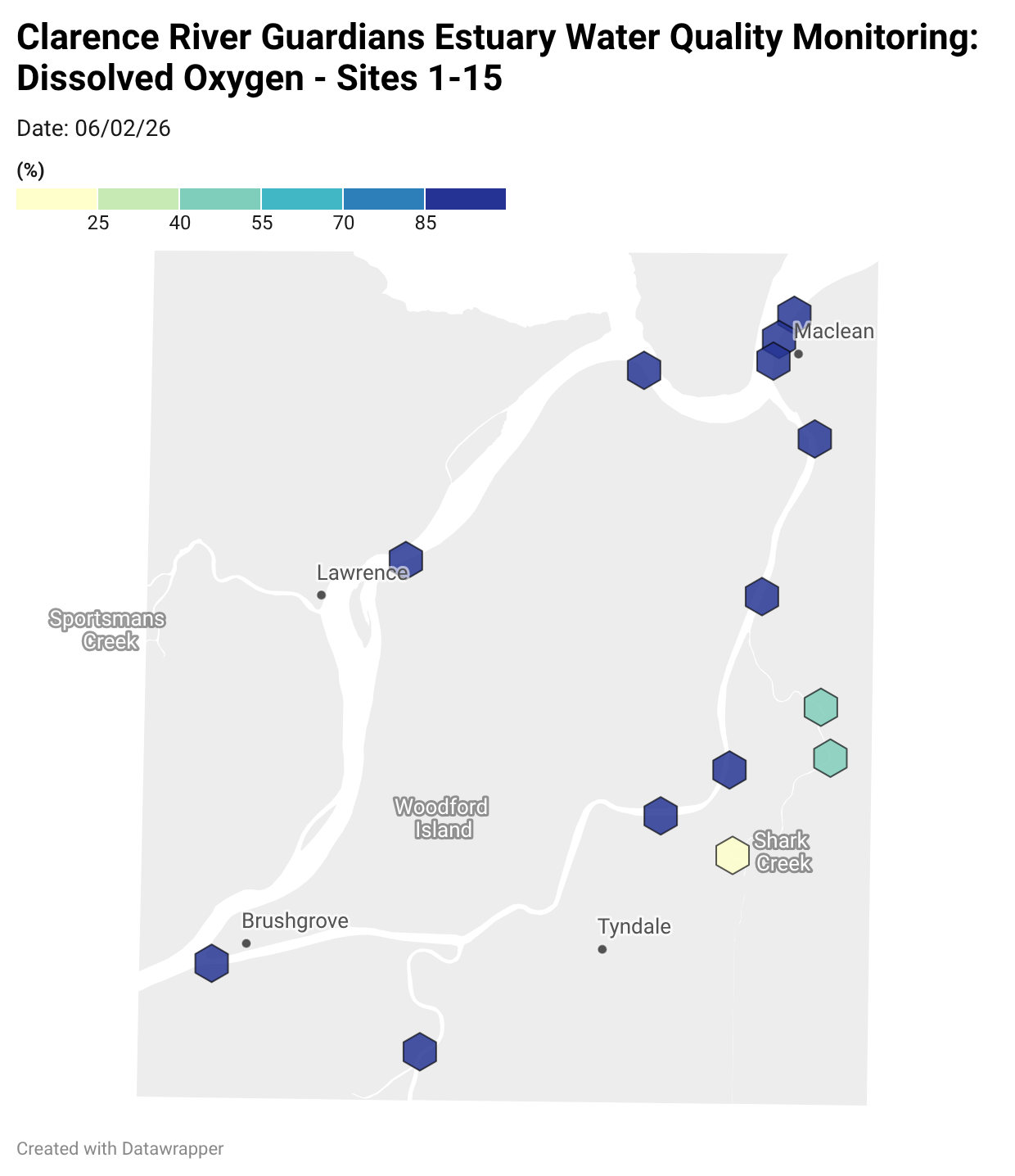
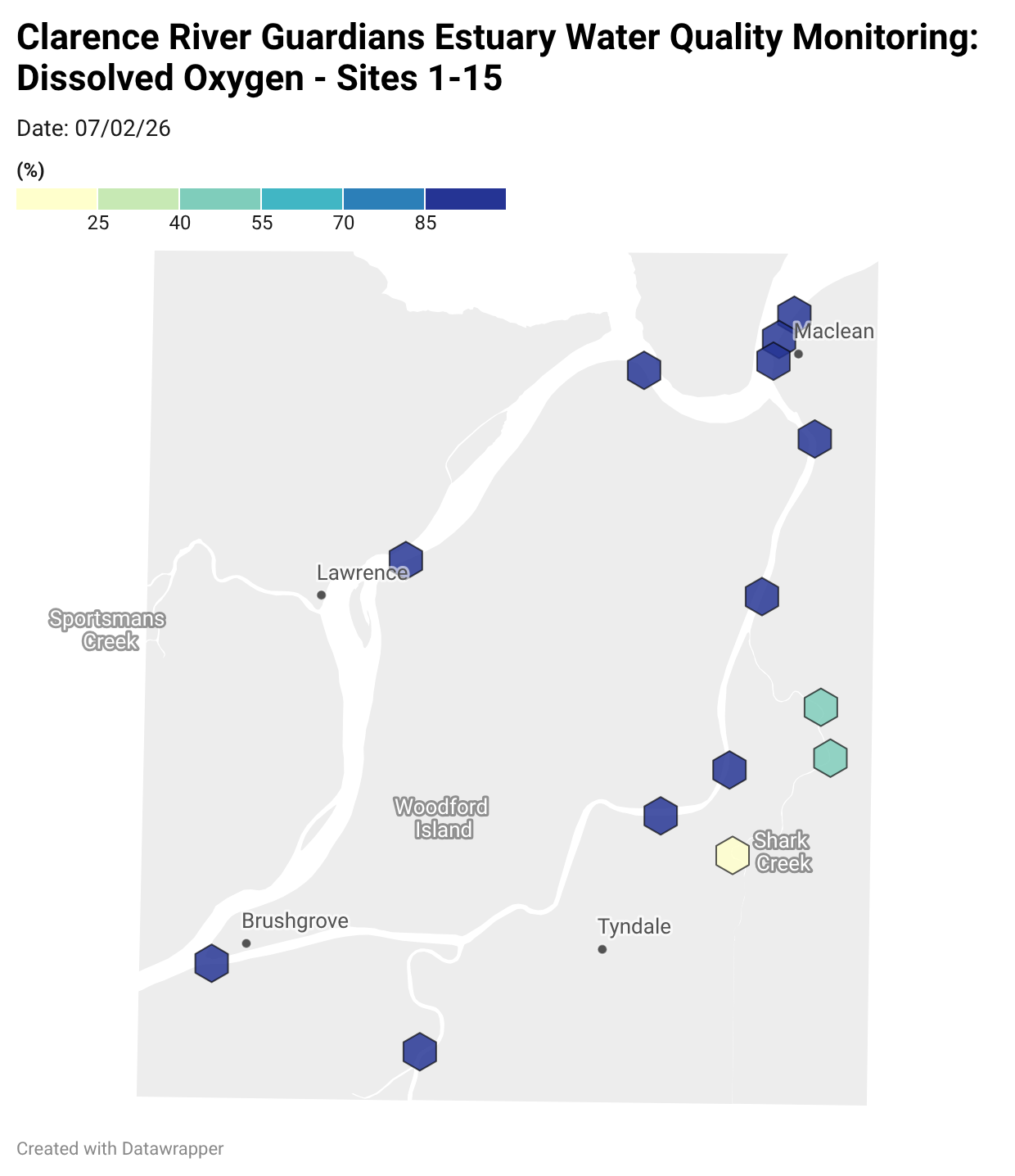
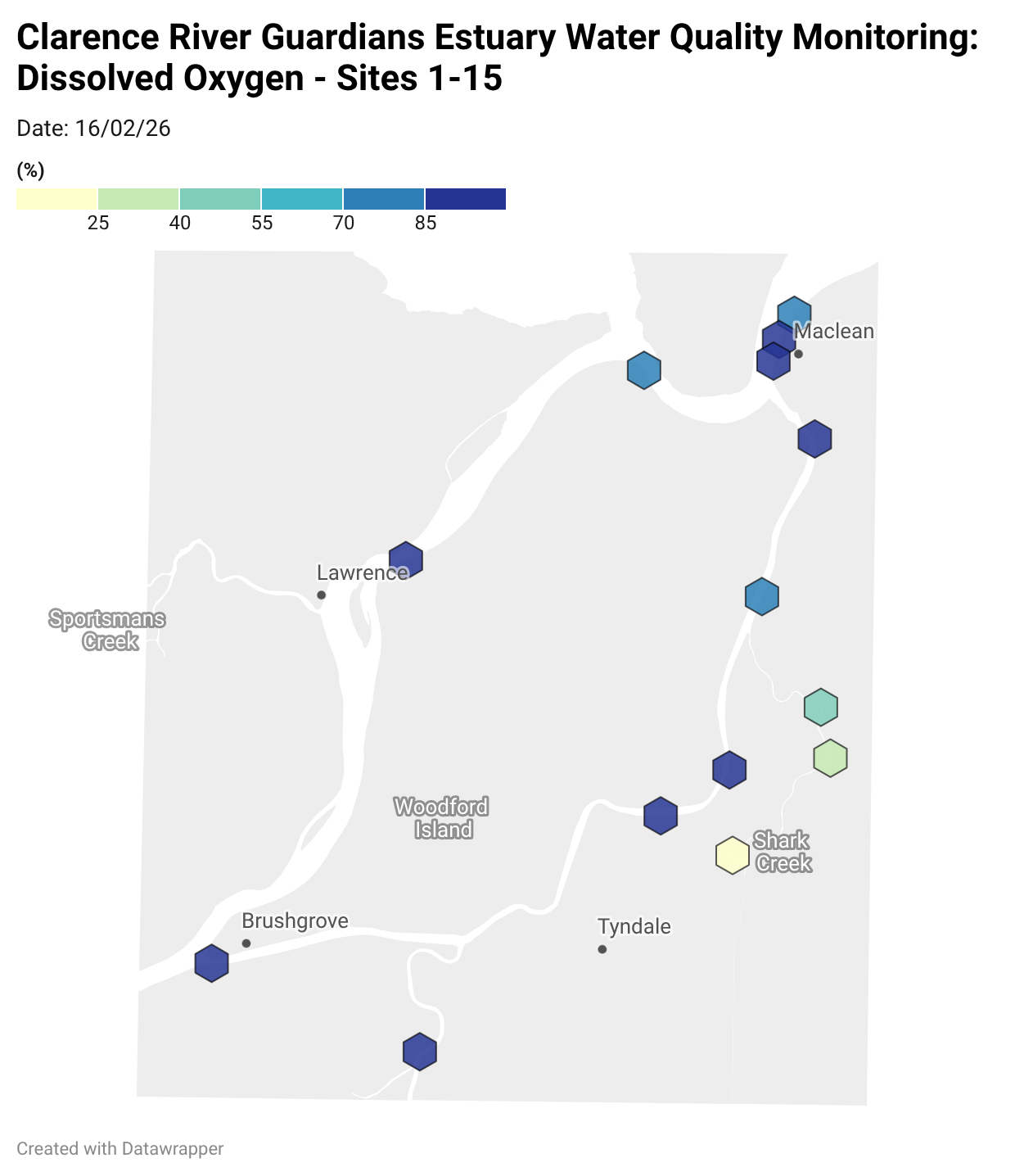
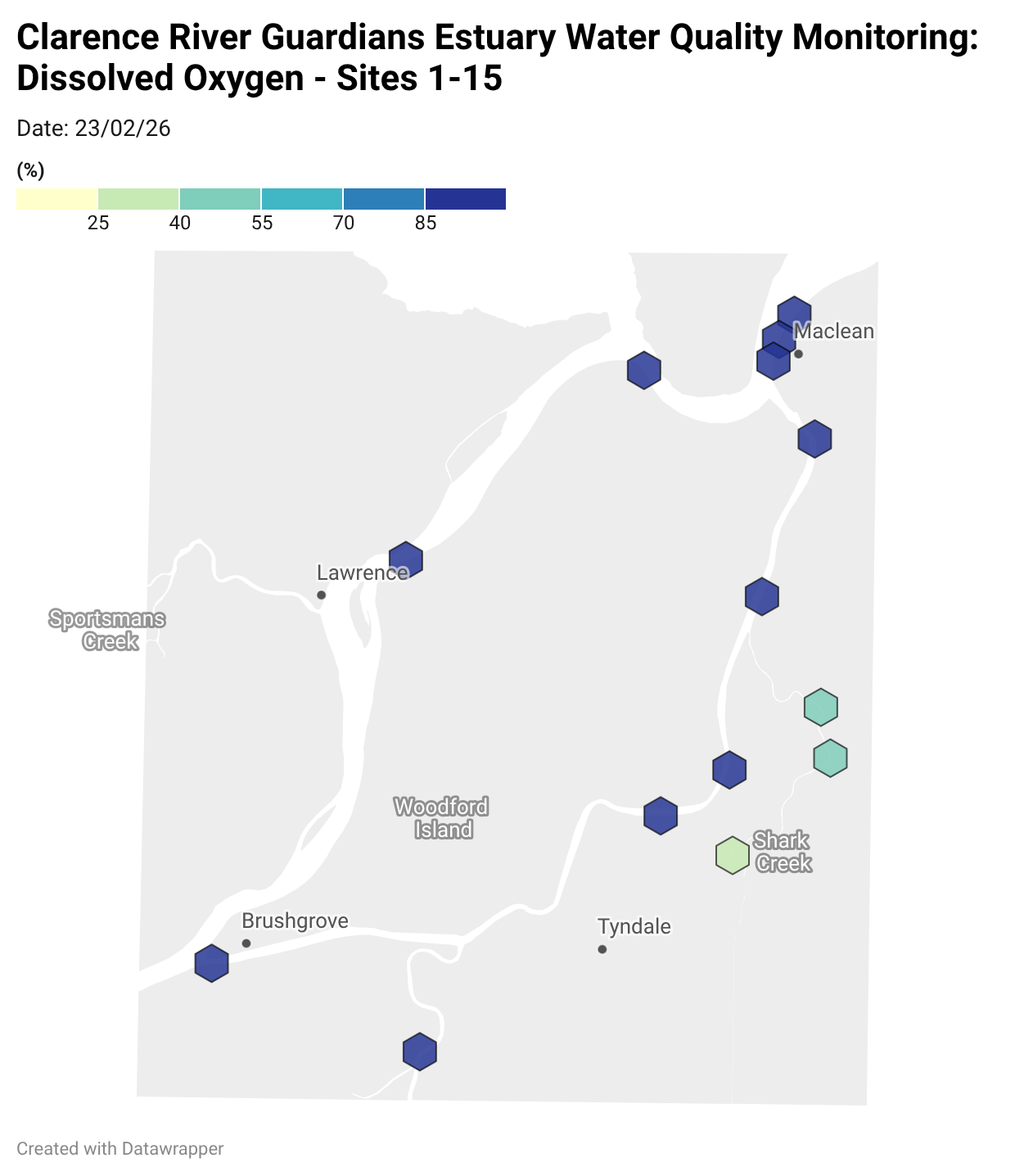
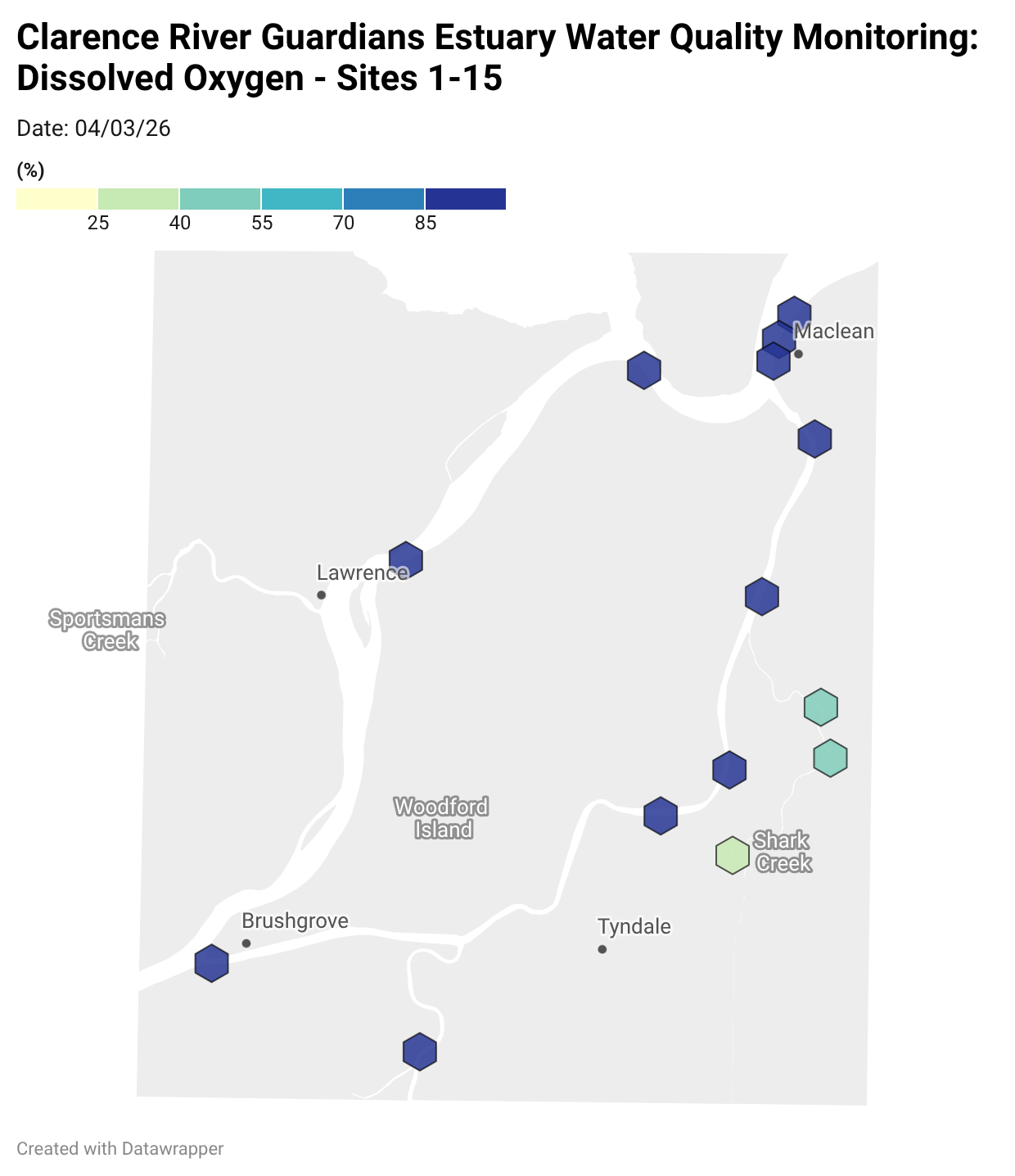
Estuary water quality monitoring locations are shown in the map. The current focus is the middle estuary, spanning from Brushgrove (upstream) to near Maclean (downstream), and brackets the South Arm Channel, Shark Creek and the Coldstream. Monitoring occurs ~approximately weekly.
Water quality monitoring locations in the Clarence Estuary.
What are we monitoring? - Dissolved Oxygen
What it is:
Dissolved oxygen (DO) is the amount of oxygen available in the water for aquatic organisms to breathe. DO saturation is expressed as a percentage (%) of the maximum amount water can hold at a given temperature.
The amount of dissolved oxygen in water is influenced by temperature and salinity. Cold, fresh water holds more oxygen than warm, salty water.
Why it matters:
Fish, crabs, worms, molluscs, prawns and other aquatic life all need dissolved oxygen to survive.
Low oxygen levels can lead to stress or death for these organisms. Different species have different tolerance ranges.
Normal range:
In healthy estuarine waters, DO saturation is typically ~80–120%.
Critical thresholds:
Below 60%: May cause stress to sensitive species.
Below ~30%: Can lead to fish kills and dead zones.
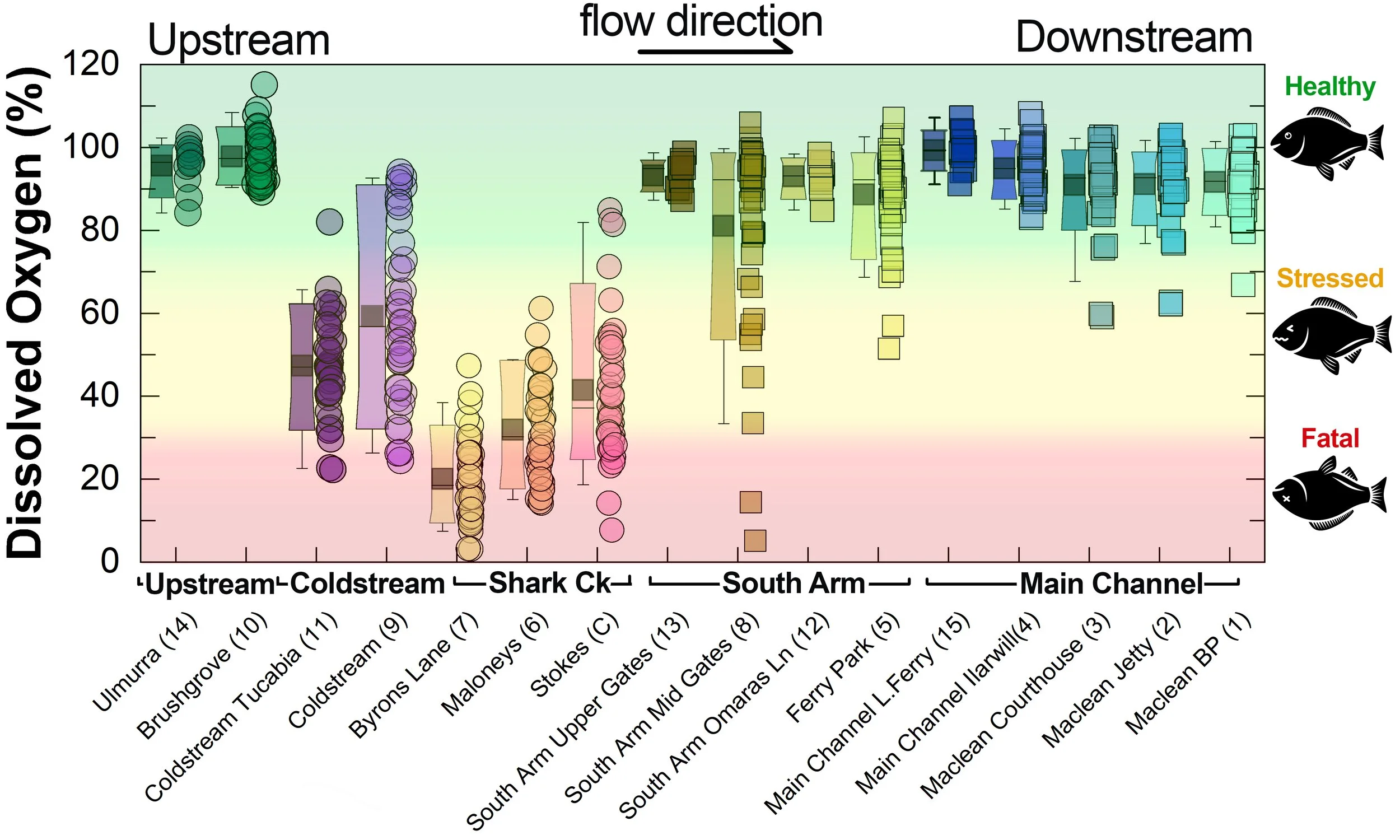
Summary of all dissolved oxygen data collected at monitoring sites from June 2025** [Updated 04/03/26]. **Note: Sites 12, 13, 14 and 15 monitored since December 2025 only.
What are we monitoring? - pH
What it is:
pH measures how acidic or basic the water is, on a scale from 0 (very acidic) to 14 (very basic). A pH of 7 is neutral. pH is in a log-scale - each pH unit decrease represents a 10x increase in acidity.
Why it matters:
Most aquatic organisms are adapted to live within a narrow pH range. Changes in pH can affect their health and the availability of nutrients and metals.
Normal range:
Estuarine waters usually have a pH between ~6.9 and 8.2.
Critical thresholds:
Below 6.5 or above 9.0: Can be harmful to aquatic life and disrupt biological processes. Different species have different tolerance ranges.
Juvenile fish, larvae and fry are generally more sensitive to low pH water.
Acidic water can cause stress and sub-lethal impacts that increase fish susceptibility to disease (i.e. redspot or EUS).
Below about pH 5.5, dissolved aluminium can become elevated in water. Dissolved aluminium can be highly toxic to aquatic organisms at very low concentrations.
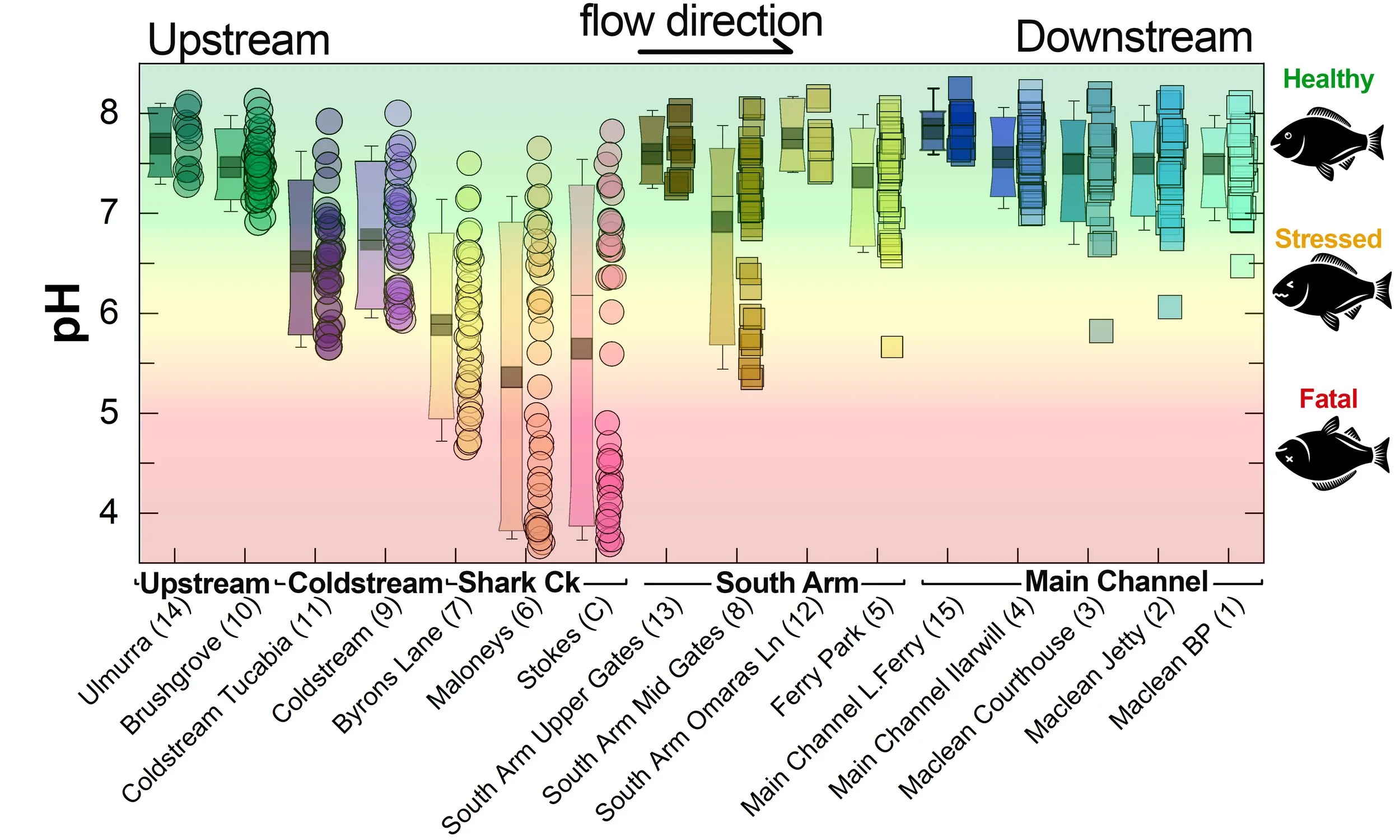
Summary of all pH data collected at monitoring sites from June 2025** [Updated 04/03/26]. **Note: Sites 12, 13, 14 and 15 monitored since December 2025 only.
What are we finding?
Persistent low dissolved oxygen and low pH in Shark Creek and the Coldstream
The data clearly shows that both Dissolved Oxygen and pH in parts of Shark Creek and the Coldstream are often in the stressful, and sometimes fatal, range for fish and other aquatic life. This water can then negatively impact the South Arm channel (see section below for a detailed snapshot).
Both oxygen and pH values tend to be persistently lower when conditions are wet and water is actively draining from the floodplain drains - especially from wetlands with acid sulfate soils.
The values tend to improve during dry conditions as drainage stops and salty water from the ocean pushes further up the estuary - as this provides both dilution and buffering.
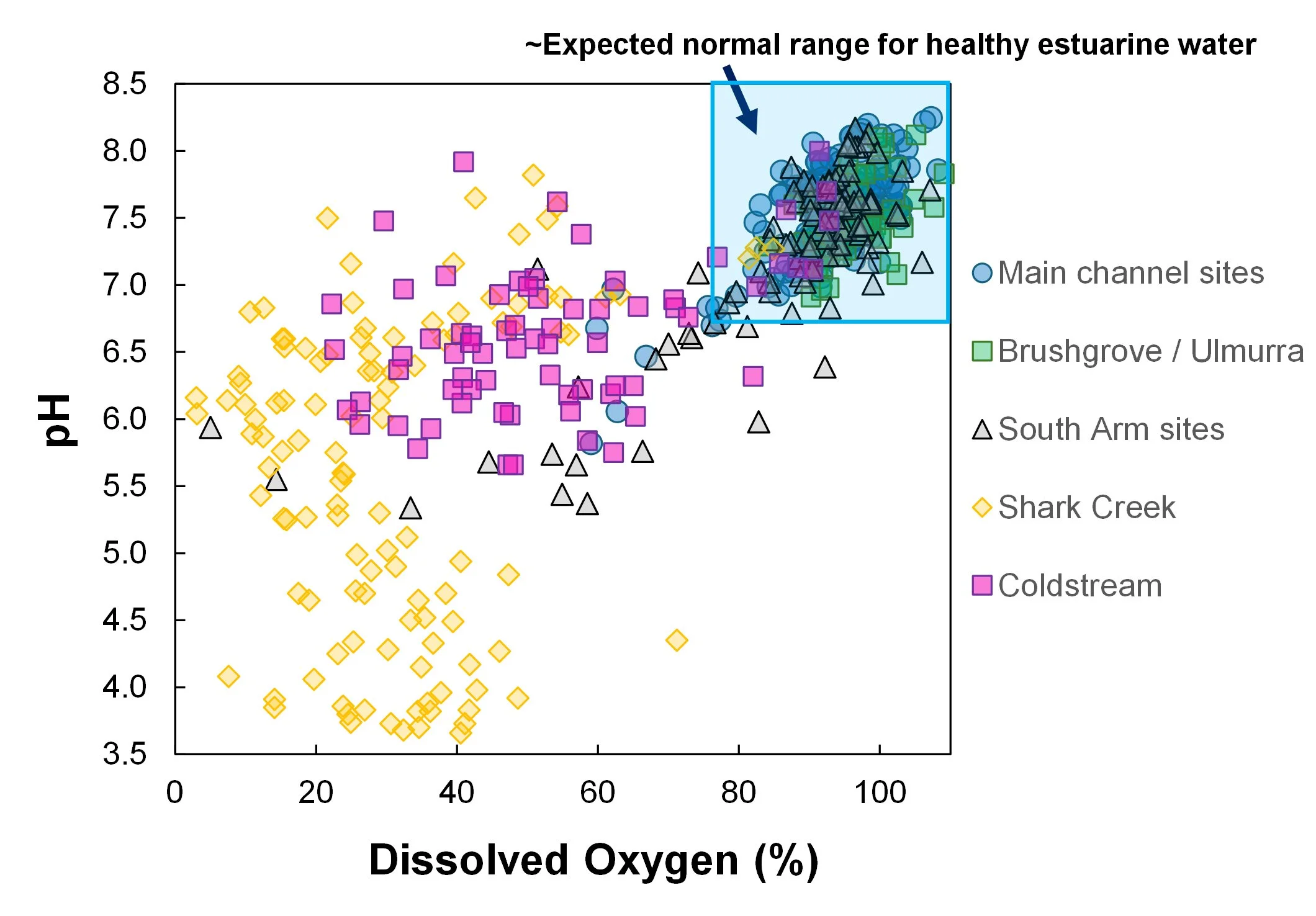
Summary of dissolved oxygen vs pH grouped according to location (data from June 2025 to Feb 2026). The blue box shows the range that would be expected for normal, healthy estuarine water.
[Healthy Estuarine waters usually have a pH between ~6.9 and 8.2 and a dissolved oxygen saturation is between ~80–120%.]
Animation of changes in pH and dissolved oxygen over time at four sites (Main Channel; Coldstream; Shark Creek; Byrons Lane):
June till February 2026
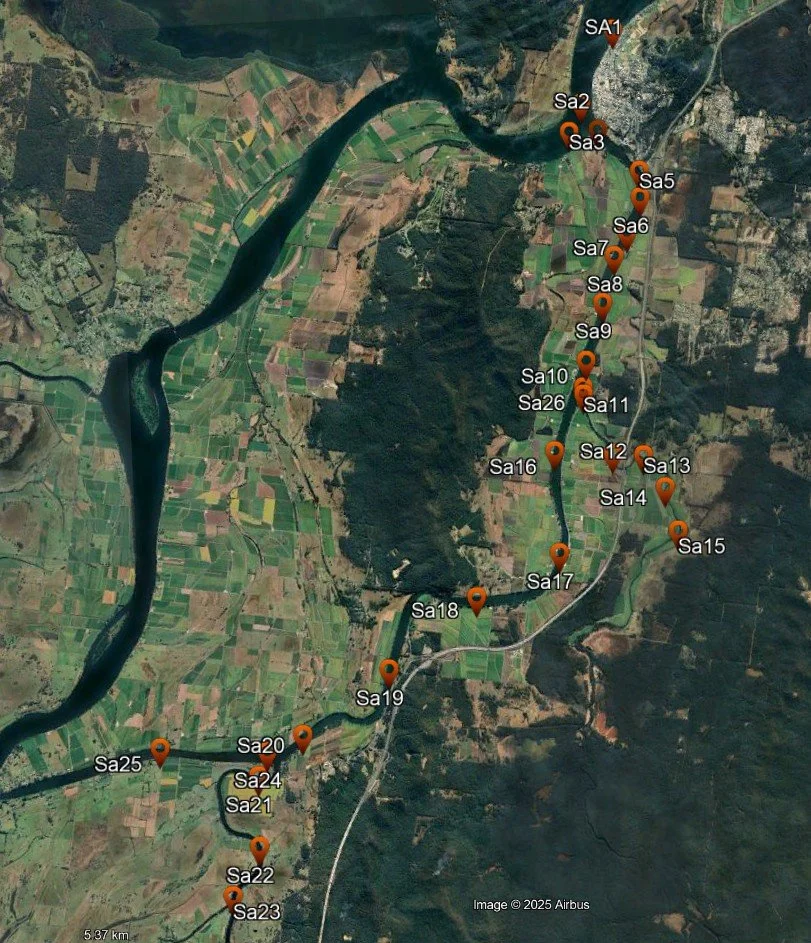
In late September 2025, a Team of trained volunteers in conjunction with Southern Cross University conducted a boat-based survey of water quality in the South Arm, Shark Creek and the Coldstream, collecting 25 samples from Maclean to near Brushgrove (Figure 1).
In addition to normal monitoring of water quality, samples were collected and taken to Southern Cross Laboratories for detailed analysis of trace metals and major ions. Samples were collected with permission from Yaegl Traditional Owners and Custodians.
The purpose was to better map the extent of water quality deterioration in this part of the estuary.
A detailed snapshot of the South Arm
Figure 1: Map of sample locations
Figure 2 shows data from this survey in relation to distance upstream along the South Arm (measured from the first sample point at Maclean).
The Main Clarence Channel sites are blue, South Arm sites are brown and Shark Creek and the Coldstream branches are shown in pink and green respectively.
Junctions between the South Arm and Shark Creek and the Coldstream are shown by arrows and text.
Plotting the data
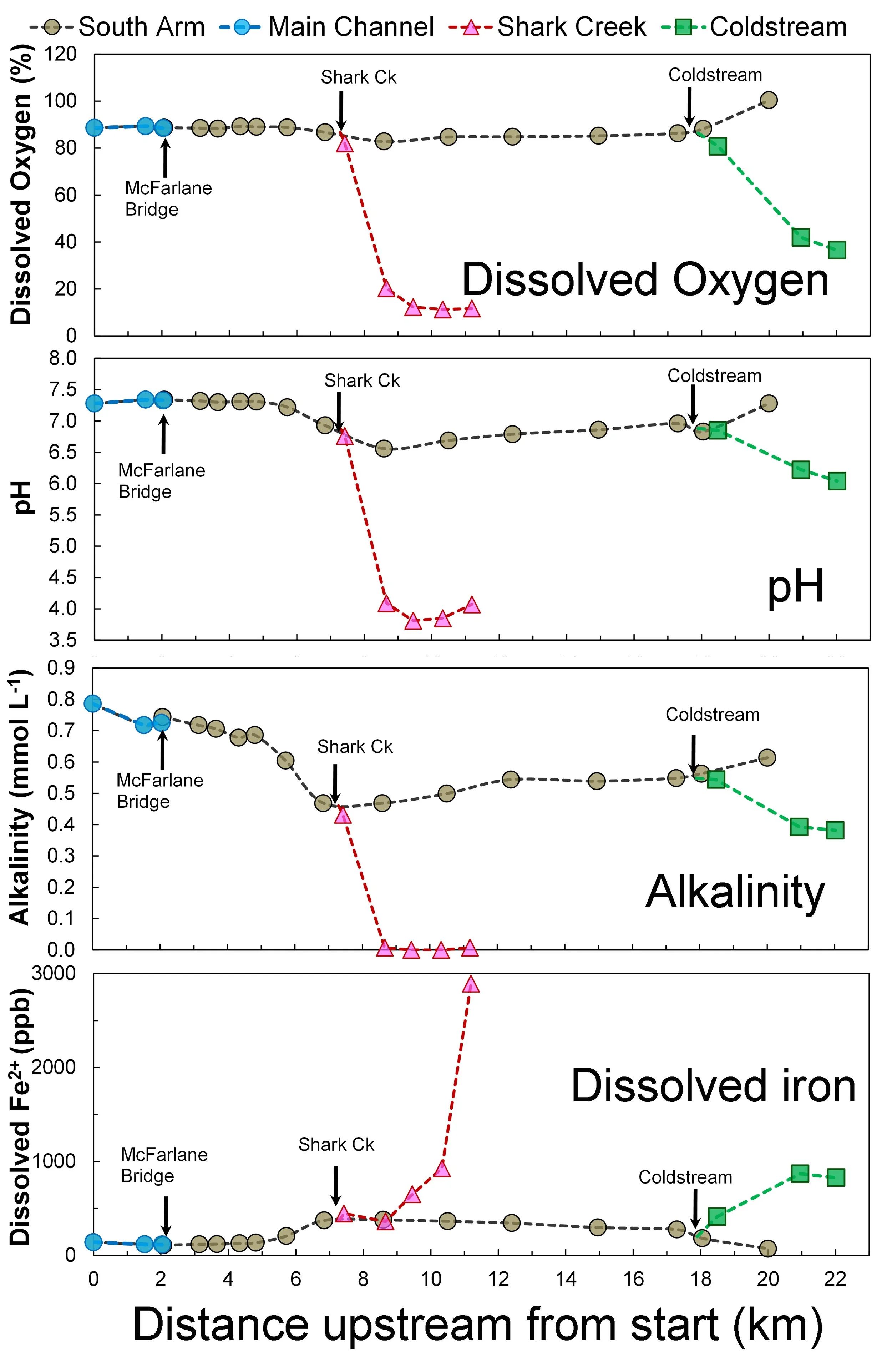
Figure 2: Changes in dissolved oxygen, pH, alkalinity and dissolved iron 2+ in relation to distance upstream
It is clear that both Shark Creek and the Coldstream are both focal points and sources of poor-quality water (Figure 2).
The low dissolved oxygen, low alkalinity, elevated iron and high acidity (low pH) - especially in Shark Creek and the Coldstream – is readily apparent (Figure 2).
These observations are all consistent with inputs of artificial drainage waters from modified floodplain wetlands with acid sulfate soils.
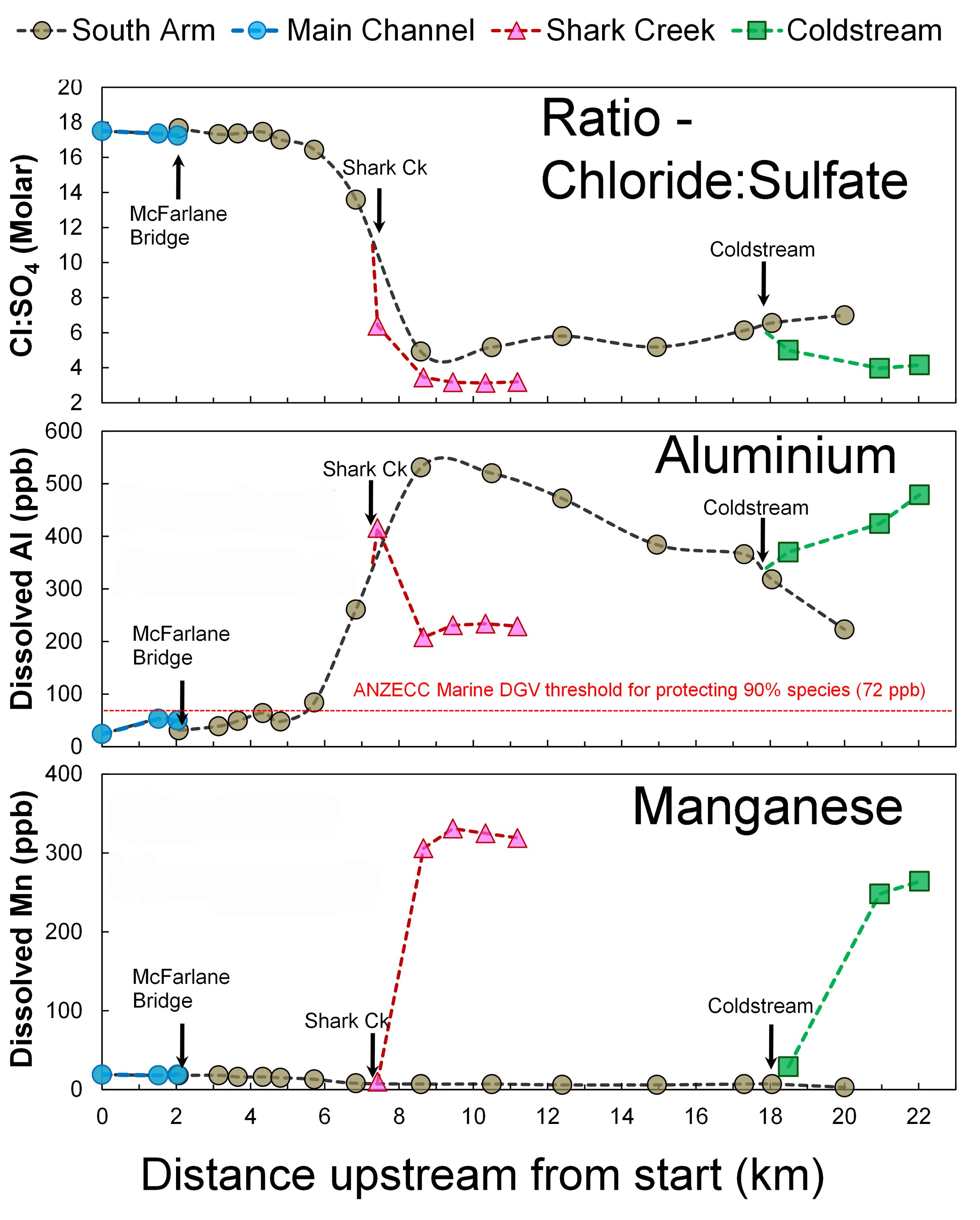
Unexpectedly, parts of the South Arm, Shark Creek and Coldstream had highly elevated dissolved aluminium (Figure 3).
Multiple locations exceeded the ANZECC Water Quality guideline threshold value of 72 ppb dissolved aluminium for protection of 90% of marine species, with maximum recorded concentrations >400 ppb (parts per billion).
Distinctly elevated (above normal background) dissolved Manganese (Mn) was also evident in both Shark Creek and the Coldstream (Figure 3).
Notably, there was also a sharp decline in the Chloride:Sulfate ratios, right near the confluence with Shark Creek. This indicates there is an additional source of sulfate into estuarine waters in the South Arm, Shark Creek and Coldstream (Figure 3).
This is a clear and unmistakable fingerprint of acid sulfate soil runoff and demonstrates that the elevated dissolved metals (Aluminium, Iron, Manganese) in this part of the estuary are due to artificial over-drainage of floodplain wetlands with acid sulfate soils.
Importantly, the effects of acid sulfate soils on water chemistry in the South Arm appears to persist for many weeks after rainfall has ceased.
Further community monitoring will be conducted in 2026.
What did we find? - High acid, elevated metals and low oxygen
Figure 3: Changes in chloride:sulfate ratios, dissolved aluminium and dissolved manganese in relation to distance upstream.
Results from community water quality monitoring (pH and dissolved oxygen) in the estuary are regularly updated and displayed below.
These maps show changes over time on a colour scale - toggle through to see different dates.